In vitro induction amplification, freeze preservation and anabiosis method of immune cells
An immune cell and cryopreservation technology, applied in the biological field, can solve the problems of natural killer cell culture, cryopreservation and recovery methods that need to be improved, expansion multiples, poor tumor killing effect, complicated culture system, etc., to achieve good cell characteristics , low cost and high induction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
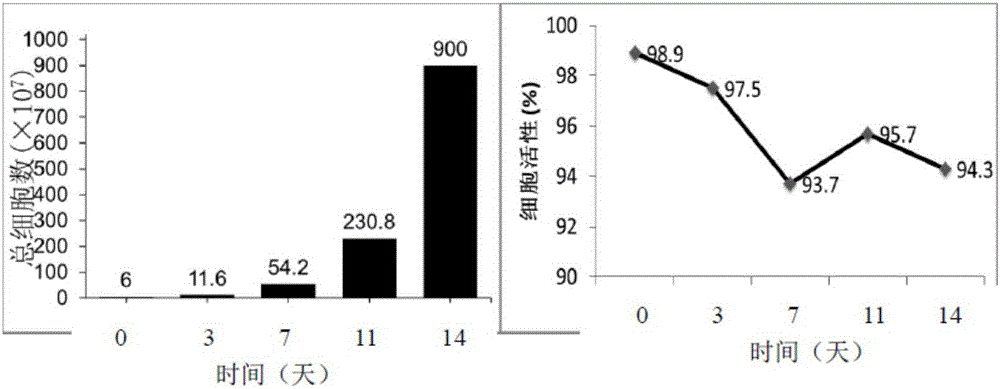
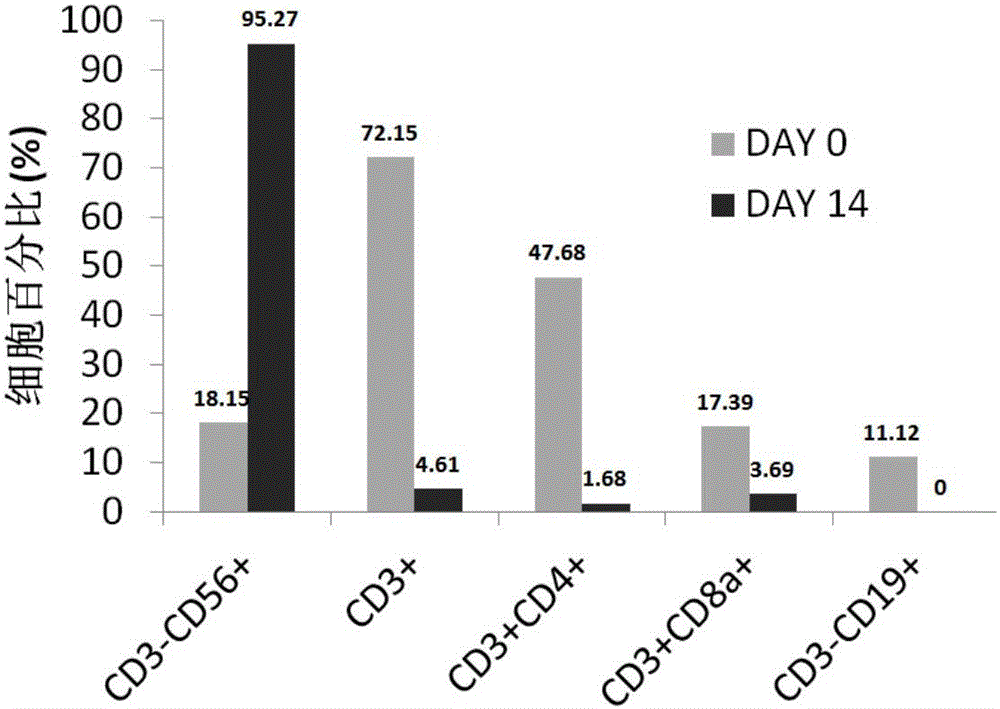
[0087] Using the method for inducing and expanding immune cells of the embodiment of the present invention, the isolated mononuclear cells are induced and expanded into immune cells, and the activity of the immune cells is detected.
[0088] 1. Experimental method
[0089] 1. Prepare T75 bottles coated with anti-human CD16
[0090] 1.1 Add 5 mL of 2.5 μg / mL anti-human CD16 monoclonal antibody dissolved in medical physiological saline to a sterile culture flask, gently shake the culture flask to spread the antibody over the culture surface, and avoid light overnight at 4°C.
[0091] 1.2 Recover the antibody coating solution before use, wash the culture flask once with 5 mL of saline, and then use 5 mL of T cell expansion medium (OpTmizer TM CTS TM T-cell expansion SFM) wash once.
[0092] 2. Collect peripheral blood, separate peripheral blood plasma and mononuclear cells
[0093] 2.1 Use a sterile blood collection bag with anticoagulant to collect about 100ml of human peripheral blood. R...
Embodiment 2
[0136] In this example, six cryopreservation solutions containing plasma and six cryopreservation solutions containing plasma were used to cryopreserve the NK cells induced and expanded in vitro in Example 1, and compare the effects of cryopreservation to observe the effect of plasma on The effect of cryopreservation of NK cells, and reducing the DMSO concentration to reduce the possibility of cytotoxicity. details as follows:
[0137] 1. Freeze NK cells with cryopreservation liquid without plasma
[0138] 1.1. Methods of cryopreservation and recovery of NK cells:
[0139] The NK cells obtained on the 10th day of the in vitro induction and expansion of Example 1 were cryopreserved in four freezing solutions containing HSA, and the components of the four freezing solutions were as follows:
[0140] Cryopreservation solution 1: 5 vol% DMSO + 10 vol% HSA;
[0141] Cryopreservation solution 2: 5 vol% DMSO + 15 vol% HSA;
[0142] Cryopreservation solution 3: 10% by volume DMSO+10% by volume...
Embodiment 3
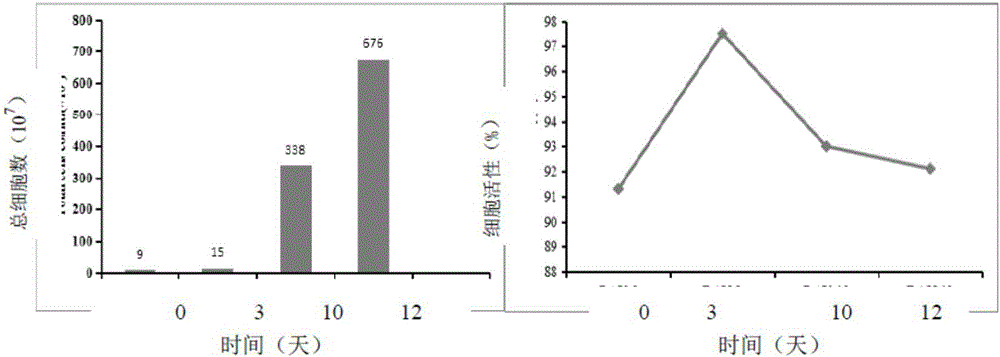
[0184] The NK cells cryopreserved in the cryopreservation solution 9 of Example 2 were recovered, and the specific method was as follows
[0185] 1. The recovery process
[0186] (1) Take out the NK cells cryopreserved in the cryopreservation solution 7 of Example 2 in liquid nitrogen, and quickly melt them in a 37-42°C water bath.
[0187] (2) The cells were treated with an equal volume of X-VIVO15 medium (denoted as group P) and an equal volume of resuscitation solution containing 2.5% HSA and 5% Dextran 40 (denoted as F1 group).
[0188] (3) Centrifuge, take the cells separately, count them with a cell viability analyzer, and measure the cell viability.
[0189] 2. Training after resuscitation
[0190] The cells of group P and group F1 were cultured in vitro using immune cell scale expansion medium, namely SuperCulture TML500 human lymphocyte serum-free medium + final concentration of 1000IU / ml domestic IL-2, and cell technology and Vitality analysis.
[0191] 3. Calculation
[0192] S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com