In-situ gel preparation and use thereof
An in-situ gel and preparation technology, applied in aerosol delivery, medical preparations of non-active ingredients, extracellular fluid diseases, etc., can solve problems affecting drug absorption and curative effect, shortening the contact time between drugs and nasal cavity surfaces, etc. , to achieve the effect of no nasal cilia toxicity, good compliance and strong affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Preparation formula and preparation method:
[0012]
[0013] Preparation method: Weigh the prescription amount of Poloxamer 188 and hydroxypropylmethylcellulose K4M, add 600mL of deionized water, heat in a water bath at 90°C to completely dissolve the material aqueous solution into an environmentally sensitive hydrophilic gel, and then add the prescription The amount of polyethylene glycol 600, glucose, and Bropol to dissolve and mix well, add deionized water to the total amount, and divide into nasal drops or nasal spray bottles with spray pumps to obtain the product.
Embodiment 2
[0015] Preparation formula and preparation method:
[0016]
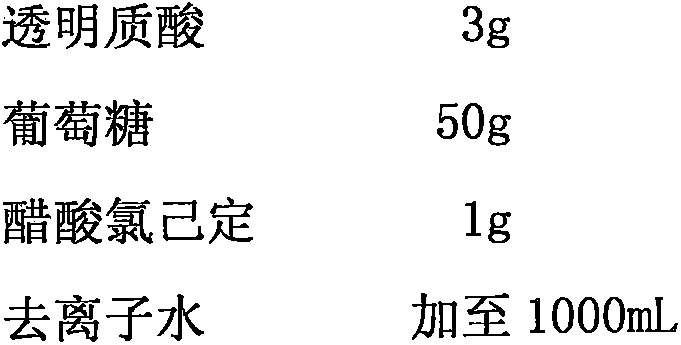
[0017]
[0018] Preparation method: Weigh the prescribed amount of deacetylated gellan gum and Carbomer 910, add 800mL of deionized water, heat in a 90°C water bath to completely dissolve the material aqueous solution into an environmentally sensitive hydrophilic gel, and then add the prescribed amount of Dissolve hyaluronic acid, glucose, and chlorhexidine acetate, mix well, add deionized water to the total amount, and divide into nasal drops or nasal spray bottles with a spray pump to obtain the product.
Embodiment 3
[0020] Preparation formula and preparation method:
[0021]
[0022] Preparation method: Weigh the prescribed amount of Poloxamer 407 and Carbomer 940, add 650mL of deionized water to shake and mix, refrigerate in a refrigerator at 4°C overnight to fully swell to form a uniform and clear gel solution, and then add Prescribed amount of polyethylene glycol 200, mannitol, benzalkonium bromide, benzoic acid to dissolve and mix thoroughly, add deionized water to the total amount, and divide into nasal drops or nasal spray bottles with spray pumps , that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com