CeO2@MnOx low-temperature SCR flue gas denitrification catalyst as well as preparation method thereof and application thereof
A denitration catalyst and flue gas technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc. The low temperature does not meet the problems of the catalyst's optimal activity temperature and heating flue gas, and achieves excellent low-temperature catalytic performance, improved anti-toxicity, increased specific surface area and acidity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
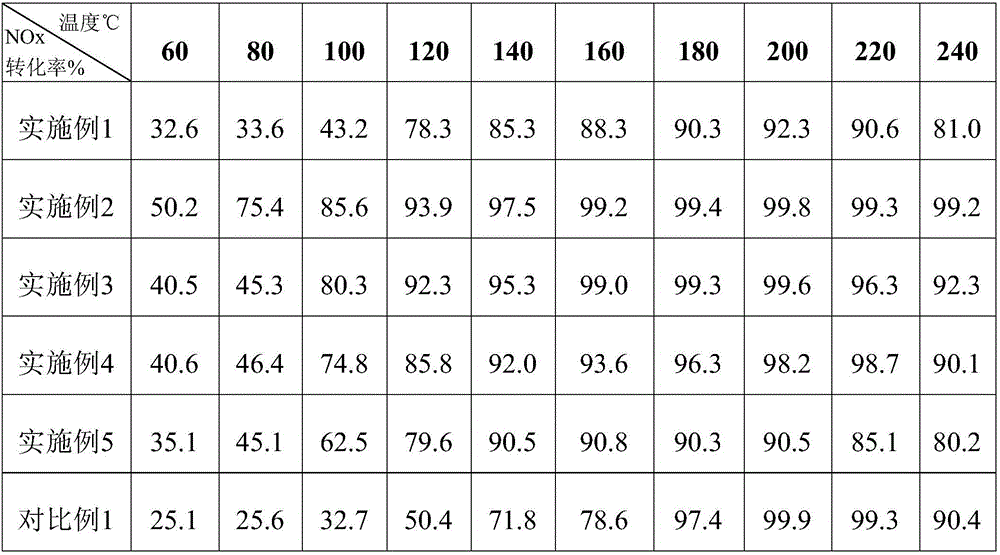
Examples
Embodiment 1
[0035] (1) Solution configuration: Weigh 2.45g of inorganic manganese source (manganese acetate tetrahydrate, purity greater than 99%) and fully dissolve in 80mL deionized water, and fully stir for 10min to obtain solution A; weigh 0.0125g PEG6000 (purity greater than 99%) was dissolved in 30mL deionized water, and fully stirred for 10min to obtain solution B; weigh 3mL NH 4 .OH (purity greater than 99%) was dissolved in 60mL deionized water, and fully stirred for 10min to obtain solution C;
[0036] (2)MnO xFormation: Add solution B and solution C dropwise to solution A obtained in step (1) successively, and fully stir and react for 1.5h, the reaction product is washed and dried at 60°C for 12h to obtain a solid powder;
[0037] (3) MnO x Roasting: the powder obtained in step (2) was placed in a tube furnace, and heated at 0.5°C·min in an air atmosphere -1 Roast at 400°C for 4h, cool to room temperature to get MnO x nanoparticles;
[0038] (4)CeO 2 @MnO x Preparation: ...
Embodiment 2
[0042] (1) Solution configuration: Weigh 2.45g of inorganic manganese source (manganese acetate tetrahydrate, purity greater than 99%) and fully dissolve in 80mL deionized water, and fully stir for 10min to obtain solution A; weigh 0.0125g PEG6000 (purity greater than 99%) was dissolved in 30mL deionized water, and fully stirred for 10min to obtain solution B; weigh 5mL NH 4 .OH (purity greater than 99%) was dissolved in 60mL deionized water, and fully stirred for 10min to obtain solution C;
[0043] (2) MnO x Formation: Add solution B and solution C dropwise to solution A obtained in step (1) successively, and fully stir the reaction for 2 hours, the reaction product is washed and dried at 60°C for 12 hours to obtain a solid powder;
[0044] (3) MnO x Roasting: put the powder obtained in step (2) in a tube furnace, and heat it at 1°C·min in an air atmosphere -1 Roast at 500°C for 3h, cool to room temperature to get MnO x nanoparticles;
[0045] (4)CeO 2 @MnO x Preparat...
Embodiment 3
[0049] (1) Solution configuration: Weigh 2.45g of inorganic manganese source (manganese acetate tetrahydrate, purity greater than 99%) and fully dissolve in 80mL deionized water, and fully stir for 10min to obtain solution A; weigh 0.0125g PEG6000 (purity greater than 99%) was dissolved in 30mL deionized water, and fully stirred for 10min to obtain solution B; weigh 5mL NH 4 .OH (purity greater than 99%) was dissolved in 60mL deionized water, and fully stirred for 10min to obtain solution C;
[0050] (2) MnO x Formation: Add solution B and solution C dropwise to solution A obtained in step (1) successively, and fully stir and react for 1.8h, the reaction product is washed and dried at 60°C for 10h to obtain a solid powder;
[0051] (3) MnO x Roasting: put the powder obtained in step (2) in a tube furnace, and heat it at 1°C·min in an air atmosphere -1 Roast at 500°C for 2h, cool to room temperature to get MnO x nanoparticles;
[0052] (4)CeO 2 @MnO x Preparation: Weigh ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com