Composite metal oxide loading active metal catalyst and preparation method thereof
A technology of active metals and composite metals, applied in the direction of metal/metal oxide/metal hydroxide catalysts, carbon compound catalysts, physical/chemical process catalysts, etc., to achieve the effect of simple process, high dispersion and uniform dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A. Add 3.8460g Mg(NO 3 ) 2 ·6H 2 O and 3.6000g Ti(SO 4 ) 2 Dissolve in 70mL of deionized water to prepare a mixed metal salt solution.
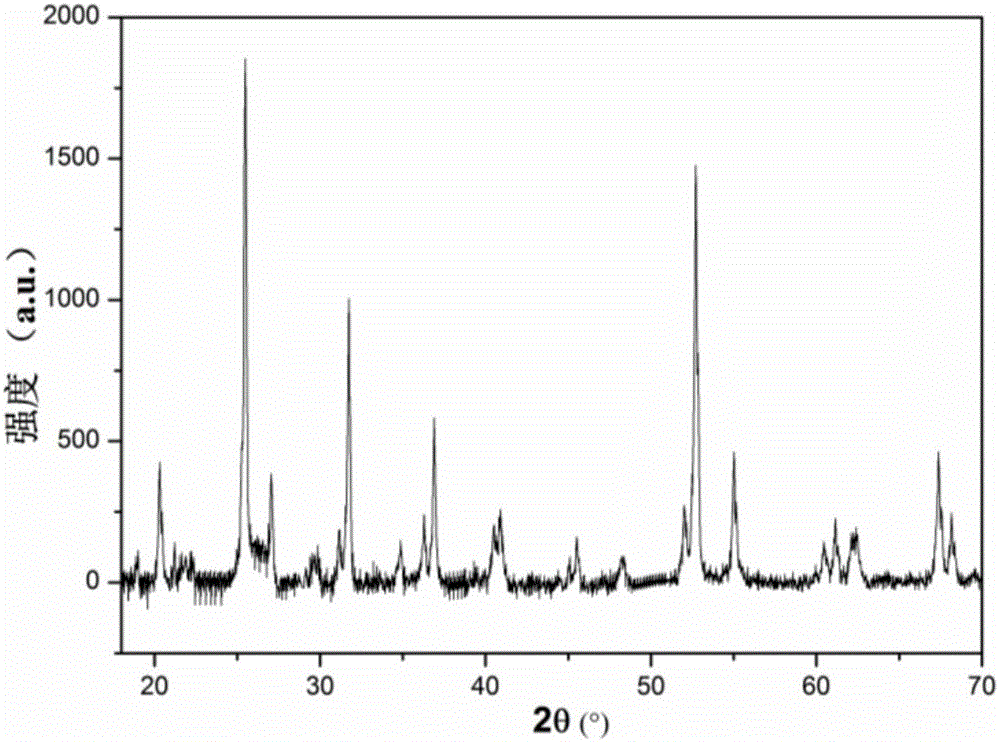
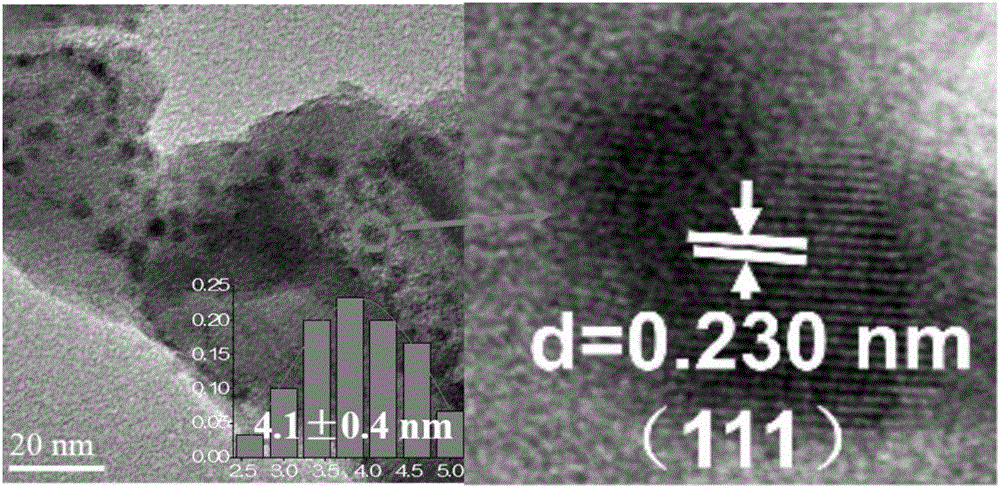
[0038] B. Dissolve 6.1950g of urea into the mixed metal salt solution prepared in step A, and transfer the uniformly dissolved solution to a 100mL reactor, and react in an oven at 150°C for 6h. After the reaction was completed, it was lowered to room temperature, and the obtained product was centrifuged, washed to neutrality, dried, and heated at 10°C·min in an air atmosphere. -1 The rate is raised to 450°C for calcination and kept for 4h to obtain Mg with a microsphere structure 5 Ti 5 o x The carrier has an average diameter of 1.8 μm.
[0039] C. Weigh 0.6440g PdCl 2 and 0.4250g NaCl were dissolved in deionized water and adjusted to 100mL to prepare Na 2 PdCl 4 solution; weigh 0.8495g AgNO 3 Dissolve in deionized water and dilute to 100mL to prepare AgNO 3 solution.
[0040] D. Add 560μL 36.3mmol / L Na 2 PdCl 4 solutio...
Embodiment 2
[0045]A. Add 2.5640g Mg(NO 3 ) 2 ·6H 2 O and 1.8757g Al(NO 3 ) 3 9H 2 O was dissolved in 170 mL of deionized water to prepare a mixed metal salt solution.
[0046] B. Dissolve 10g of urea into the mixed metal salt solution prepared in step A, and transfer the uniformly dissolved solution to a 250mL reactor, and react in an oven at 90°C for 24h. After the reaction was completed, it was lowered to room temperature, and the obtained product was centrifuged and washed to neutrality, then the precipitate obtained in the lower layer was dried, and the obtained precursor was heated in an air atmosphere at 5°C·min -1 The rate is raised to 600 ° C for 2 hours, and the Mg with microspheres is obtained. 2 al 1 o x The carrier has an average diameter of 1.0 μm.
[0047] C. Weigh 1g PtCl 2 Dissolve in deionized water and dilute to 100mL to prepare H 2 PtCl 4 solution.
[0048] D. Add 600μL 20mmol / L H 2 PtCl 4 The solution was added to 150 μL of deionized water, and 0.2 g of ...
Embodiment 3
[0051] A. Add 1.4505g Ni(NO 3 ) 2 ·6H 2 O, 0.9378g Cu(NO 3 ) 2 ·3H 2 O and 1.8757g Al(NO 3 ) 3 9H 2 O was dissolved in 70 mL of deionized water to prepare a mixed metal salt solution.
[0052] B. Dilute 7.1556g Na 2 CO 3 Dissolve in the mixed metal salt solution prepared in step A, and transfer the uniformly dissolved solution to a 100mL reactor, and react in an oven at 60°C for 48h. After the reaction was completed, it was lowered to room temperature, and the obtained product was centrifuged and washed to neutrality, then the precipitate obtained in the lower layer was dried, and the obtained precursor was heated in an air atmosphere at 10°C·min -1 The rate is raised to 600 ° C for 4 hours to obtain NiCuAlO with microspheres x The carrier has an average diameter of 1.2 μm.
[0053] C. Weigh 0.35g RhCl 3 ·3H 2 O was dissolved in deionized water and adjusted to 100 mL to prepare a Rh precursor solution.
[0054] D. Add 1.0ml 25mmol / L RhCl 3 The solution was adde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com