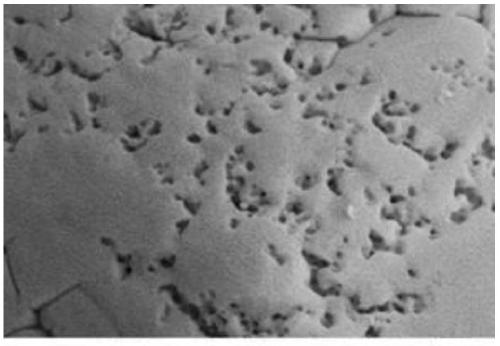
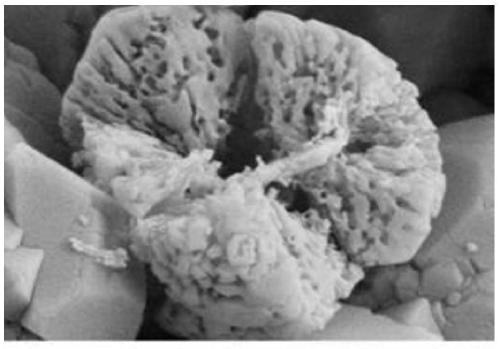
A kind of preparation method of battery grade microporous spherical iron phosphate
An iron phosphate, battery-level technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems affecting the electrical conductivity and charge and discharge performance of lithium iron phosphate, many impurities in iron phosphate, and complex preparation processes, etc., to achieve The effect of fewer types of raw materials, uniform particle size distribution, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Step 1: Measure 15mol·L -1 21.00ml of phosphoric acid solution, add FeCl 3 ·6H 2 O14.00g, and 200ml deionized water, stir and heat at 100°C for 90 minutes, and concentrate to a volume of 100ml.
[0017] Step 2: After the above solution is slightly cold, add 40ml of concentrated nitric acid with a content of 65%-68%, add 0.32g of nano silica, dilute to 1L with deionized water, and stir at a constant temperature for 3h at 80°C. Nano-silica is the crystal nucleus, causing the iron phosphate crystals to precipitate out in the solution, and then centrifugal filtering, washing and drying the precipitate to prepare battery-grade iron phosphate.
[0018] Step 3: Put the iron phosphate prepared above into 100 ml of hydrofluoric acid with a concentration of 1% and soak for 40 minutes, filter with medium-speed quantitative filter paper, wash and dry to prepare a microporous spherical battery-grade iron phosphate material.
[0019] The carbon reduction high-temperature solid-phase method ...
Embodiment 2
[0021] Step 1: Measure 15mol·L -1 21.00ml of phosphoric acid solution, add FeCl3·6H2O14.00g, and 200.00ml deionized water, stir and heat at 100°C for 90 minutes, and concentrate to a volume of 100ml.
[0022] Step 2: After the above solution is slightly cold, add 20ml of 65%-68% nitric acid and 0.32g of nano silica, dilute to 1L with deionized water, and stir at a constant temperature for 3 hours at 75°C. Silicon is the crystal nucleus, causing iron phosphate crystals to precipitate out in the solution, and then the precipitate is centrifuged, washed, and dried to prepare battery-grade iron phosphate.
[0023] Step 3: Put the iron phosphate prepared above into 100 ml of 2% hydrofluoric acid and soak for 30 minutes, filter with medium-speed quantitative filter paper, wash, and dry to prepare a microporous spherical iron phosphate material.
[0024] The carbon reduction high-temperature solid-phase method is used to further prepare lithium iron phosphate battery-grade materials, and th...
Embodiment 3
[0026] Step 1: Measure 15mol·L -1 25.00ml of phosphoric acid solution, add FeCl 3 ·6H 2 O15.00g, and 200ml deionized water, stirred and heated at 100°C for 120 minutes, concentrated to a volume of 80ml.
[0027] Step 2: After the above solution is slightly cold, add 30ml of concentrated nitric acid with a content of 65%-68%, add 0.32g of nano-silica, dilute to 1L with deionized water, and stir for 2h at a constant temperature at 80°C. Nano-silica is the crystal nucleus, which causes iron phosphate crystals to precipitate out in the solution, and then the precipitate is centrifuged, washed, and dried to prepare battery-grade iron phosphate.
[0028] Step 3: Put the above-prepared iron phosphate into 100ml of hydrofluoric acid with a concentration of 3% and soak for 30 minutes, filter with medium-speed quantitative filter paper, then wash and dry to prepare a microporous spherical battery-grade iron phosphate material.
[0029] The carbon reduction high-temperature solid-phase method i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com