Continuous synthesis system and method of ethyl 4-chloroacetoacetate
A technology for the synthesis of ethyl chloroacetoacetate, which is applied in the chemical industry, can solve problems such as difficulty in controlling the depth of chlorination, difficulty in industrial production, and difficulty in controlling the reaction, so as to reduce production raw material costs and energy costs, avoid by-products, Reduces the effect of polychlorinated products and other by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
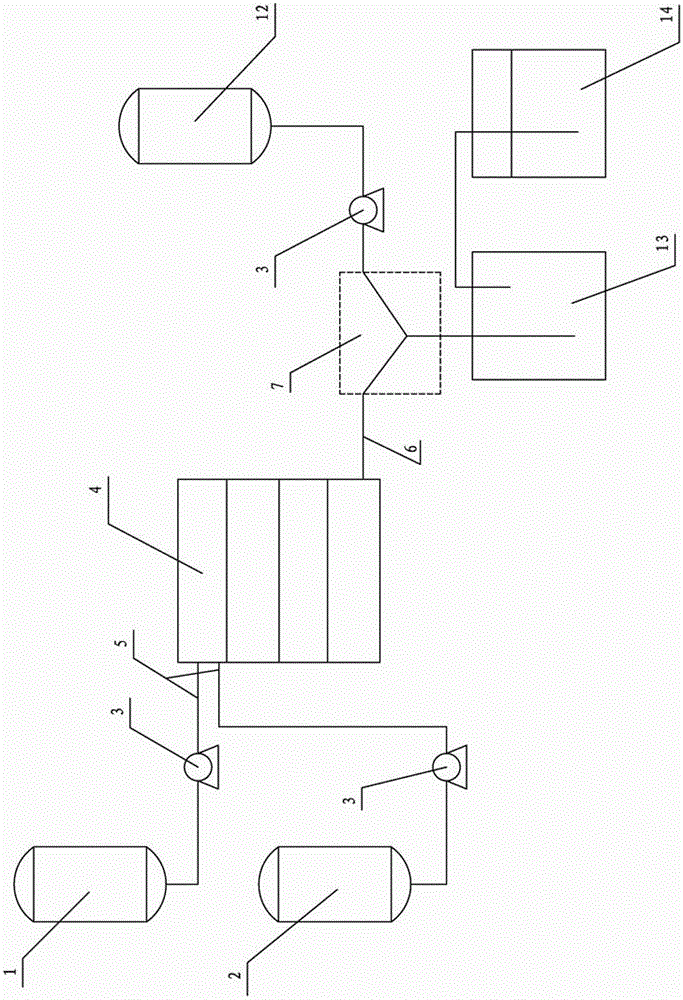
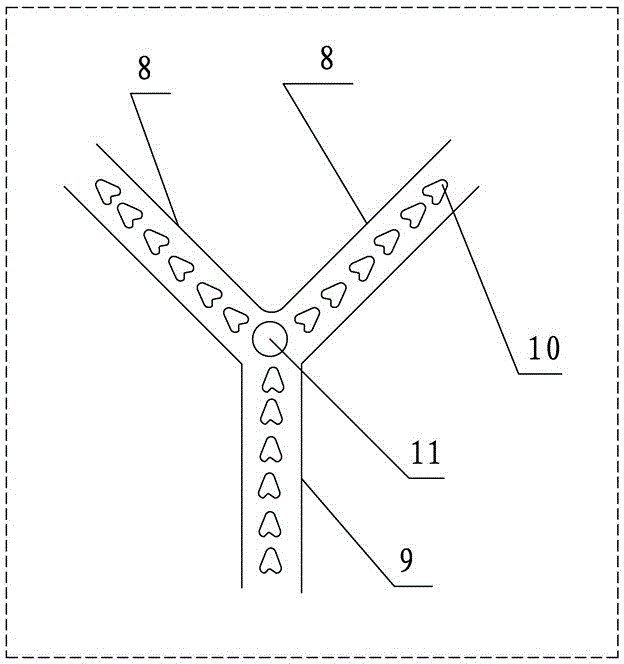
[0026] Such as figure 1 , figure 2 Shown, a kind of continuous synthesis system of ethyl chloroacetoacetate comprises the first solution tank 1, the second solution tank 2, pump 3, microchannel reactor 4; The upstream of this microchannel reactor 4 is provided with Feed pipe 5, downstream is provided with discharge pipe 6; Described first solution tank 1, the second solution tank 2 are respectively connected with the feed pipe 5 of described microchannel reactor 4 by pump 3; Also comprise the second reactor 7. The second reactor 7 includes a feed branch pipe 8 and a discharge main pipe 9. There are at least two feed branch pipes 8; a third solution tank 12 is also included, and the feed branch pipe 8 is connected to the third solution tank respectively. The solution tank 12 and the discharge pipe 6 are connected, and a pump 3 is arranged between the third solution tank 12 and the feed branch pipe 8; a collection tank 13 and an exhaust gas absorbing device 14 are also include...
Embodiment 2
[0029] A method for the continuous synthesis system of ethyl 4-chloroacetoacetate described in Example 1, mainly comprising the following steps:
[0030] 1) Diketene and chlorine gas are dissolved in organic solvent respectively to form a solution; the molar ratio of diketene to organic solvent is 0.05~50:1; the molar ratio of chlorine gas to organic solvent is 0.01~50:1;
[0031] 2) Input the prepared diketene and chlorine gas solution into the microchannel reactor 4 through pump 3 to carry out chlorination reaction; the reaction temperature range of diketene solution and chlorine gas solution is -50℃~0℃, and the reaction time is 0.1~50s The molar ratio to chlorine gas is 0.9~1.5:1;
[0032] 3) After the diketene chlorination reaction is completed, react with ethanol through the second reactor 7 to synthesize ethyl 4-chloroacetoacetate, and the product enters the collection tank 13 through the discharge main pipe 9, and the collection tank 13 is connected to the tail gas abso...
Embodiment example 3
[0037] The device used in this embodiment is the same as in Embodiment 1.
[0038] The molar ratio of diketene and methylene chloride is 0.2:1 to prepare a solution, and the molar ratio of chlorine to methylene chloride is 0.05:1 to prepare a solution;
[0039] The prepared diketene solution and chlorine gas solution were respectively input into the continuous flow microchannel reactor through metering pumps, the molar ratio of diketene to chlorine gas was 1.1:1, the reaction temperature was -5°C, and the reaction time was 30s;
[0040]After the diketene chlorination reaction is completed, the ethanol is input through the metering pump, and after the reaction is completed, it enters the product collection tank, and the collection tank is connected to the tail gas absorption device. According to GC analysis, the content of ethyl acetoacetate is 1%, the content of ethyl 4-chloroacetoacetate is 96%, the content of ethyl 2,4-dichloroacetoacetate is about 0.2%, and other impurities...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com