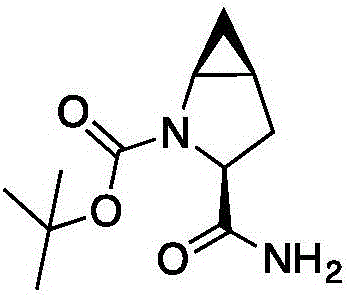
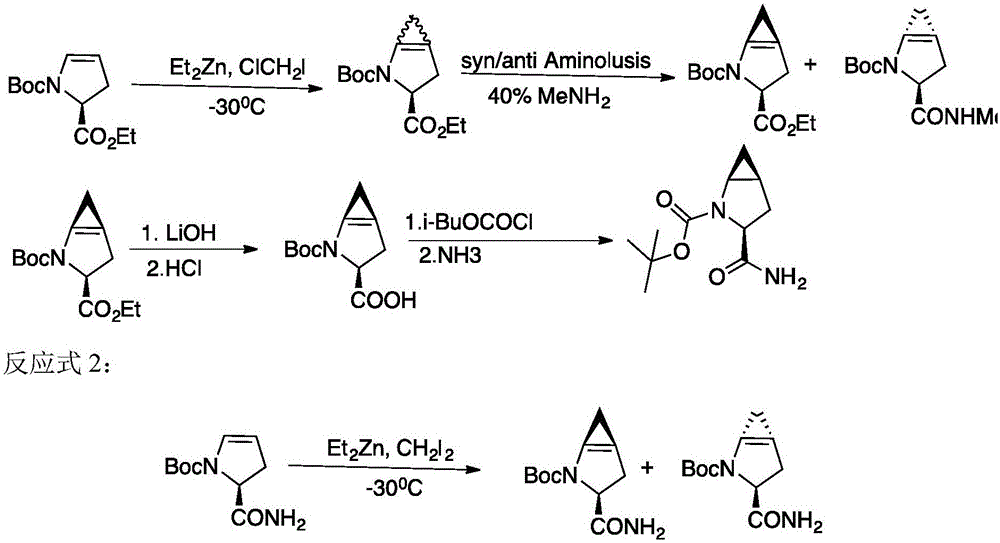
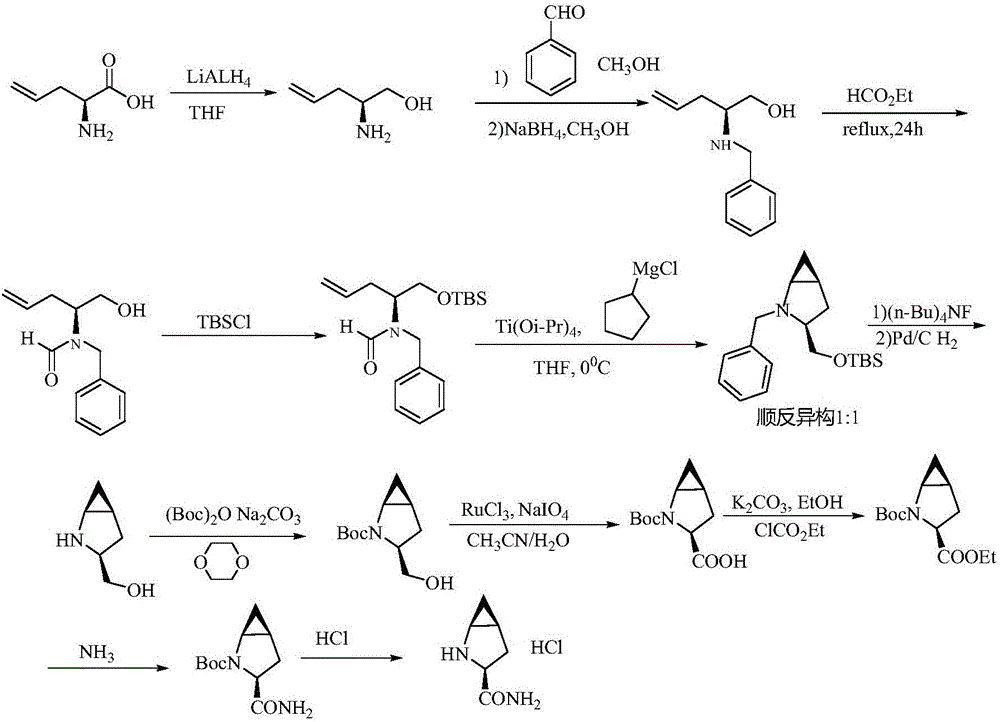
Method for preparing saxagliptin intermediate
An intermediate, reaction time technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of long route, achieve short route, high yield, no The effect of polluting the ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrolecarboxamide (5.0g, 23.6mmol) and a chiral catalyst (0.15 g) and 30.0g of dichloromethane, reacted at room temperature 25°C for 12h, filtered to remove the catalyst, and then concentrated the organic phase. After removing the solvent, the concentrate was recrystallized with ethyl acetate / n-hexane=1:2 (volume ratio) , freeze-dried after suction filtration to obtain 4.50 g (theoretical 5.33 g) of the product, the ee value was 97%, and the yield was 84.4%.
Embodiment 2
[0044]Add (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrolecarboxamide (5.0g, 23.6mmol) and the chiral Catalyst (0.1g) and 35.0g dichloromethane, after room temperature 25 ℃ reaction 12h, remove catalyst by filtration first, organic phase is concentrated again, after removing solvent, concentrate is ethyl acetate / n-hexane=1:2 (volume ratio ) recrystallization, suction filtration and freeze-drying to obtain 5.15 g (theoretical 5.33 g) of the product, the ee value was 99.5%, and the yield was 96.6%.
Embodiment 3
[0046] Add (S)-1-N-tert-butoxycarbonyl-2,3-dihydro-2-pyrrolecarboxamide (5.0g, 23.6mmol) and the chiral Catalyst (0.1g) and 20.0g dichloromethane, after reacting at 0°C for 30h, first filter to remove the catalyst, and then concentrate the organic phase. After removing the solvent, the concentrate is mixed with ethyl acetate / n-hexane=1:2 (volume ratio) After recrystallization, suction filtration and freeze-drying, 4.9 g of the product (theoretical 5.33 g) was obtained, the ee value was 98%, and the yield was 92.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com