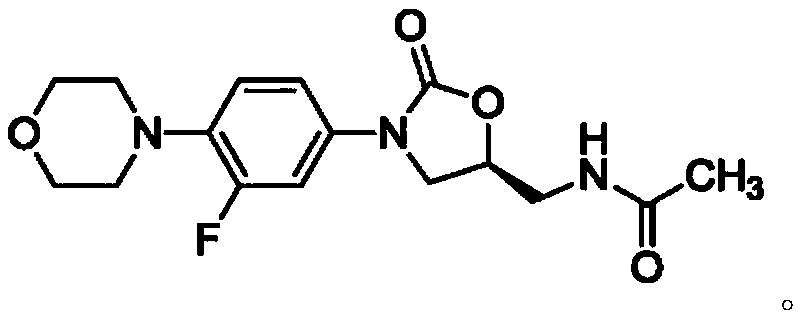
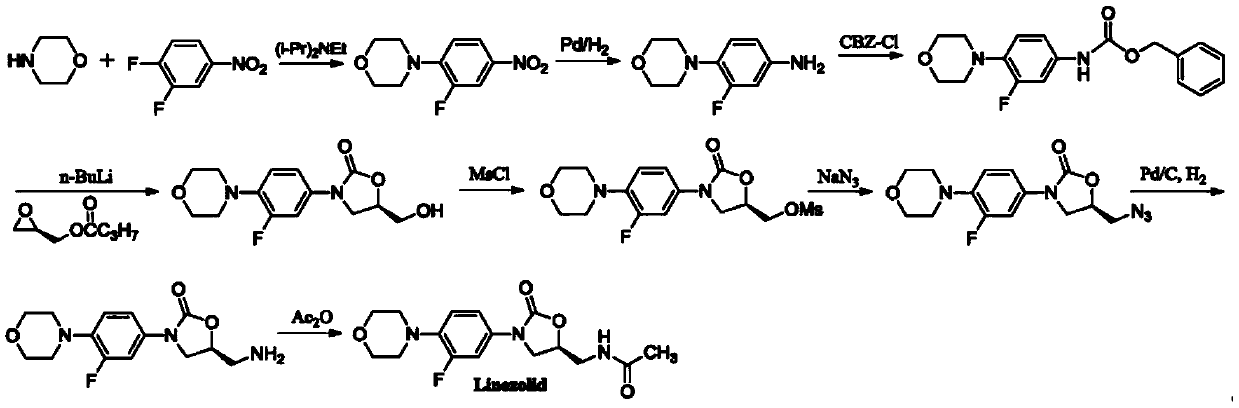
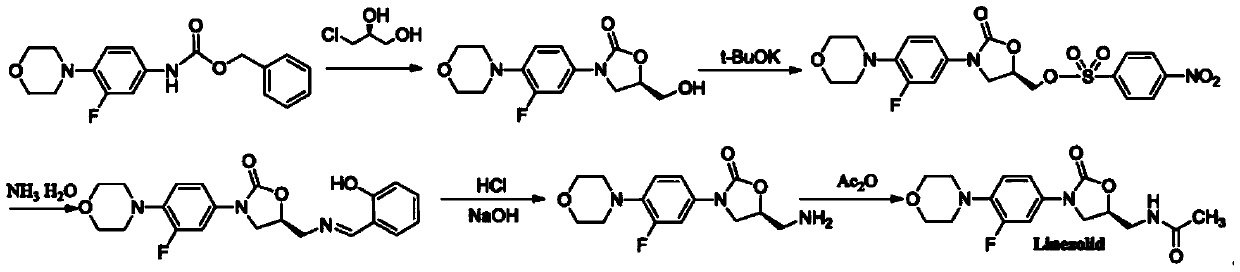
A kind of preparation method of linezolid
A technology of linezolid and phthalimide potassium salt is applied in the field of preparation of linezolid, which can solve the problem that it is difficult to meet the purity requirements of linezolid, the purification operation and the anhydrous requirements are harsh, and the atoms/processes are inconsistent. Economic and other issues, to achieve the effect of efficient preparation, high product yield and fast reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0049] Embodiment 1-1: Synthesis of Compound 3
[0050] Add phthalimide potassium salt (55.6g, 0.3mol), (S)-4-chloro-1,3-butanediol (compound 1, 37.4g, 0.3mol) and 300mLDMF into the reaction flask, heat After reacting at 80°C for 3 hours, the reaction solution was poured into 400 mL of water, stirred for 10 minutes, filtered with suction, and dried under reduced pressure to obtain 65.4 g of white solid (Compound 2), with a yield of 92.3%.
Embodiment 1-2
[0051] Embodiment 1-2: the synthesis of compound 3
[0052] Add phthalimide potassium salt (66.7g, 0.36mol), (S)-4-chloro-3-hydroxybutyronitrile (compound 1, 37.4g, 0.3mol) and 300mL DMF into the reaction flask, heat to 70 After reacting at ℃ for 5 h, the reaction solution was poured into 400 mL of water, stirred for 10 min, filtered with suction, and dried under reduced pressure to obtain 68.6 g of white solid (Compound 2), with a yield of 96.8%.
Embodiment 2-1
[0053] Embodiment 2-1: Synthesis of compound 4
[0054] in N 2 Under the condition of protection, add 47.3g of compound 2 (0.2mol) into the reaction flask with magnets, add 2000mL of ethyl acetate, put the reaction flask into an ice-water bath to cool down to 0°C, and then add PhICl 2 (218.3g, 0.8mol) and NaN 3 (104.0g, 1.6mol), the reaction was stirred at 0°C for 4h, then heated to 80°C and stirred for 8h. The reaction system was lowered to room temperature, 2000 mL of ethyl acetate was added, and it was transferred to a separatory funnel, and saturated Na 2 S 2 0 3 solution (1500mL), the separated aqueous phase was extracted with ethyl acetate (2×500mL), the combined organic phases were washed with saturated brine (500mL), anhydrous Na 2 S0 4 After drying, the organic solvent was removed by rotary evaporation to obtain 46.1 g of compound 4 with a HPLC purity of 99.2% and a yield of 93.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com