Anticancer peptide containing poly-L-histidine as well as preparation and application thereof
A technology of polyhistidine and histidine, which is applied in the field of anti-tumor polypeptide drugs, can solve the problems of increased permeability, decreased activity of cancer cells, death, etc., and achieves the effect of convenient artificial synthesis and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
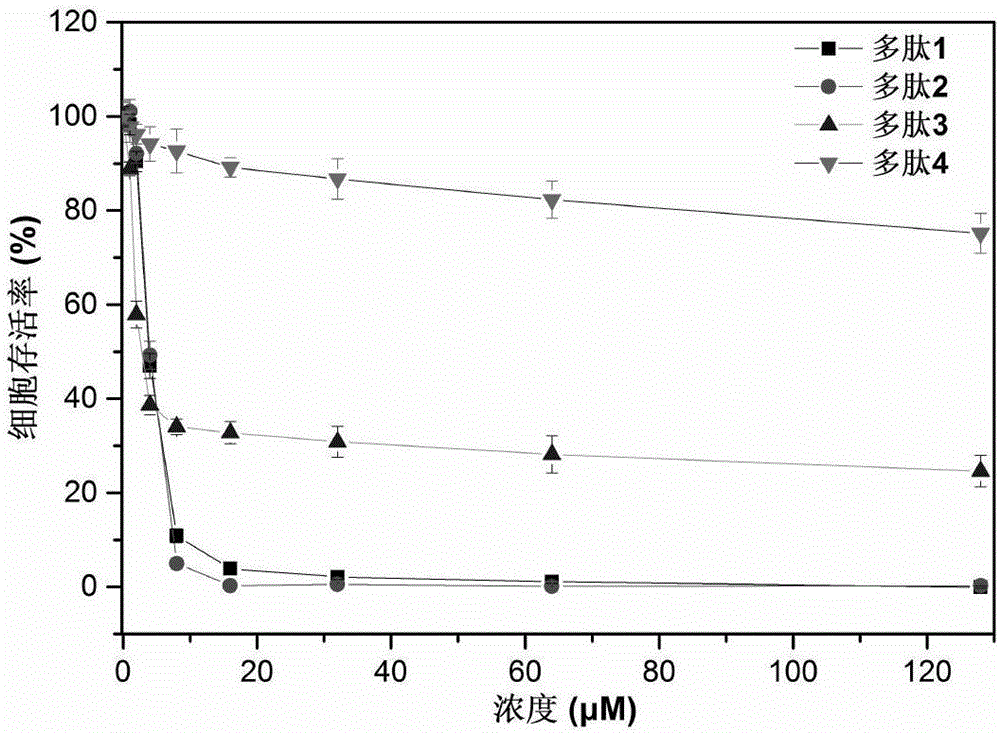
Examples
preparation Embodiment 1
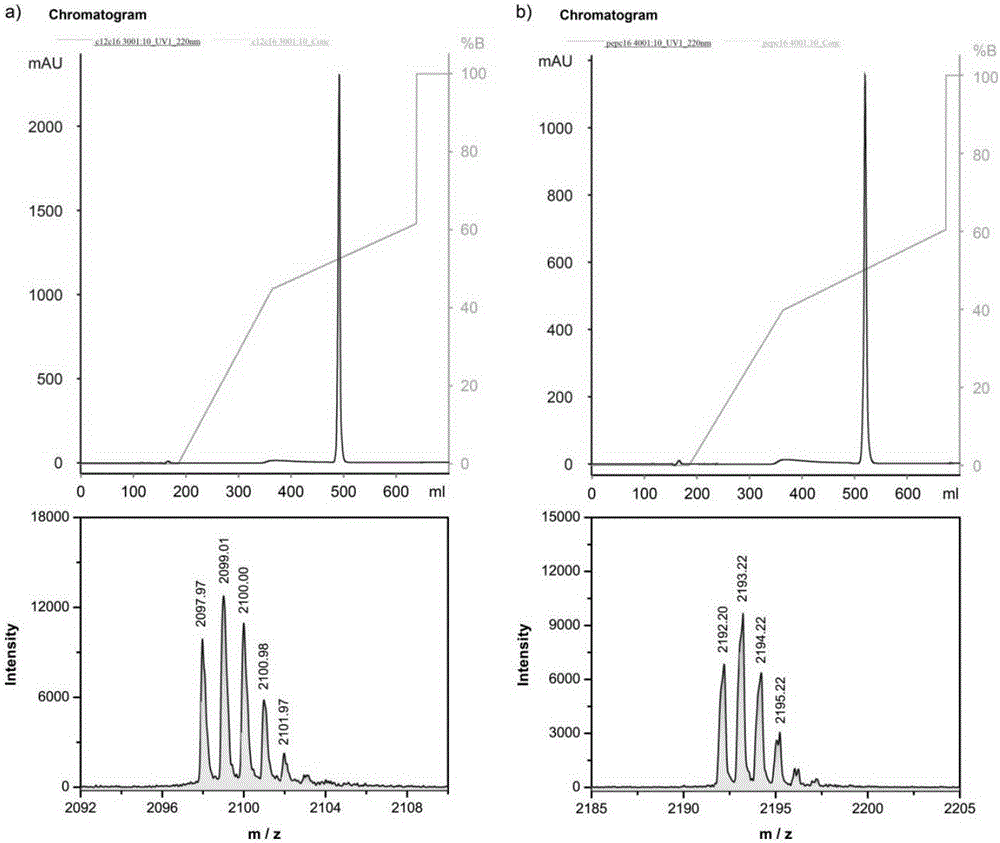
[0058] The preparation of the anticancer polypeptide 1 comprises the following steps:
[0059] (1) Add Rink amide resin (309mg, 0.810mmol / g, 0.25mmol, 1equiv) to DMF / DCM (v / v=1 / 1; 10mL) to swell for 3h, wash the resin with DMF, and then use piperidine / DMF ( v / v=1 / 4; 10mL) to remove the Fmoc protecting group, react for 20 minutes; wash the resin with DMF, put Fmoc-His(Trt)-OH (0.75mmol, 3.0equiv) in the reaction flask, add organic Alkali (2.5mmol, 10equiv) and condensing agent (0.75mmol, 3.0equiv), after reacting in DMF solution for 3 hours, remove Fmoc protecting group again, obtain the resin coupled with histidine;
[0060] (2) Put the Rink amide resin reacted with the first histidine and Fmoc-His(Trt)-OH (0.75mmol, 3.0equiv) in a reaction flask, add organic base (2.5mmol, 10equiv) and condensation The mixture (0.75mmol, 3.0equiv) was reacted in DMF solution for 3 hours at room temperature. After the reaction is complete, let stand, drain the DMF, wash the resin with DMF, t...
preparation Embodiment 2
[0069] The preparation of the anticancer polypeptide 2 comprises the following steps:
[0070] (1) Add Rink amide resin (309mg, 0.810mmol / g, 0.25mmol, 1equiv) to DMF / DCM (v / v=1 / 1; 10mL) to swell for 3h, wash the resin with DMF, and then use piperidine / DMF ( v / v=1 / 4; 10mL) to remove the Fmoc protecting group, react for 20 minutes; wash the resin with DMF, put Fmoc-His(Trt)-OH (0.75mmol, 3.0equiv) in the reaction flask, add organic Alkali (2.5mmol, 10equiv) and condensing agent (0.75mmol, 3.0equiv), after reacting in DMF solution for 3 hours, remove Fmoc protecting group again, obtain the resin coupled with histidine;
[0071] (2) Put the Rink amide resin reacted with the first histidine and Fmoc-His(Trt)-OH (0.75mmol, 3.0equiv) in a reaction flask, add organic base (2.5mmol, 10equiv) and condensation The mixture (0.75mmol, 3.0equiv) was reacted in DMF solution for 3 hours at room temperature. After the reaction is complete, let stand, drain the DMF, wash the resin with DMF, t...
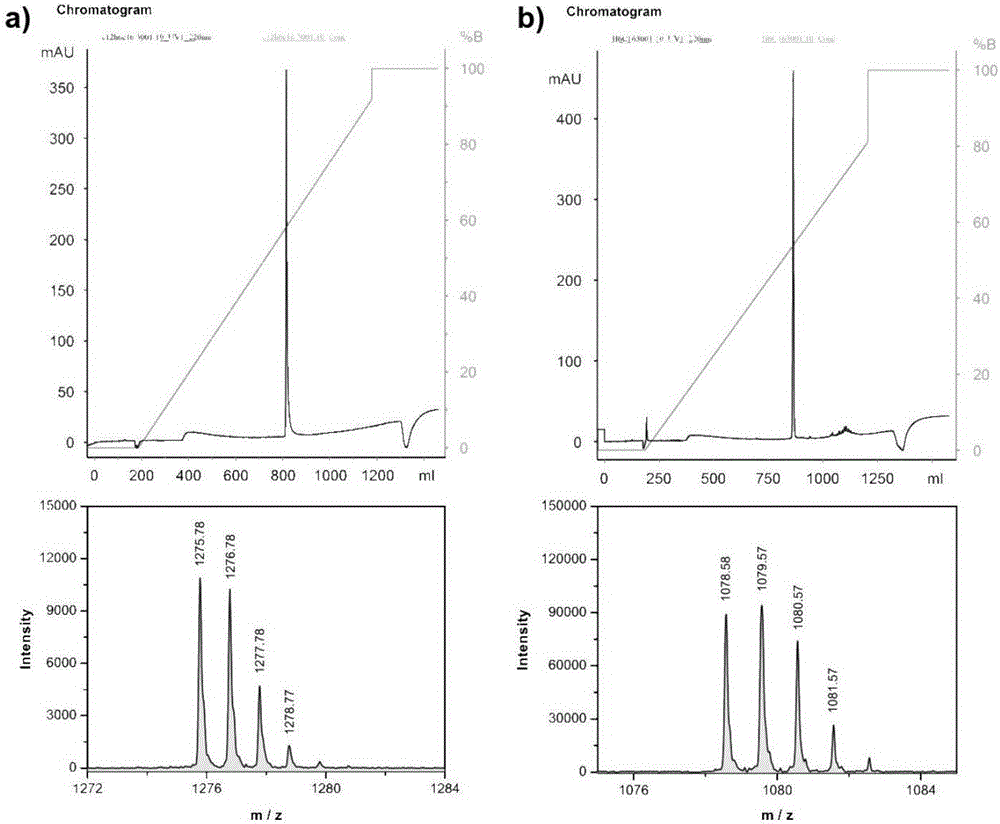
preparation Embodiment 3
[0080] The preparation of the anticancer polypeptide 3 comprises the following steps:
[0081] (1) Add Rink amide resin (309mg, 0.810mmol / g, 0.25mmol, 1equiv) to DMF / DCM (v / v=1 / 1; 10mL) to swell for 3h, wash the resin with DMF, and then use piperidine / DMF ( v / v=1 / 4; 10mL) to remove the Fmoc protecting group, and reacted for 20 minutes; the resin was washed with DMF, and the aminododecanoic acid protected by Fmoc was placed in a reaction flask, and an organic base (2.5mmol, 10equiv) was added and Condensing agent (0.75mmol, 3.0equiv), after reacting in DMF solution for 3 hours, remove Fmoc protecting group again, obtain the resin coupled with aminododecanoic acid;
[0082] (2) Place the Rink amide resin and Fmoc-His(Trt)-OH (0.75mmol, 3.0equiv) in the reaction flask after reacting with aminododecanoic acid, add organic base (2.5mmol, 10equiv) and condensing agent ( 0.75mmol, 3.0equiv), in DMF solution, reacted at room temperature for 3 hours. After the reaction is complete, l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com