Hepatitis C virus fusion antigen protein and application thereof
A hepatitis C virus, fusion antigen technology, applied in the direction of viral antigen components, viruses, viral peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The recombinant hepatitis C virus Core of embodiment 1 tobacco expression, the strong immunogenic region of E1 and E2 protein obtains
[0036] 1.1 Artificially synthesized full-length and truncated mutant genes of Core, E1 and E2
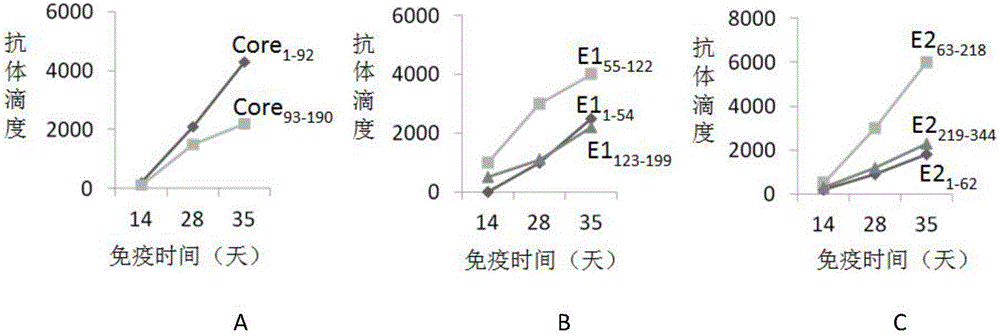
[0037] Based on the sequence of hepatitis C virus genotype 1b (see GenBank accession number HQ639947.1 for the nucleic acid sequence, and GenBank accession number AEJ86549.1 for the protein sequence) and epitope analysis software IEDB (http: / / www.iedb.org / home_v3. php) prediction, designing Core, E1 and E2 truncation mutants containing different epitopes: Core 1-92 (encodes Core protein 1-92 amino acids), Core 93-190 (encodes amino acids 93-190 of Core protein), E1 1-54 (encodes amino acids 1-54 of E1 protein), E1 55-122 (encodes amino acids 55-122 of E1 protein), E1 123-199 (encodes amino acids 123-199 of E1 protein), E2 1-62 (encodes amino acids 1-62 of E2 protein), E2 63-218 (encoding amino acid 63-218 of E2 protein) and E2 219-344 ...
Embodiment 2
[0048] Example 2 fusion antigen Core 1-92 / E1 55-122 / E2 63-218 Preparation of (HCVles) and determination of immune characteristics
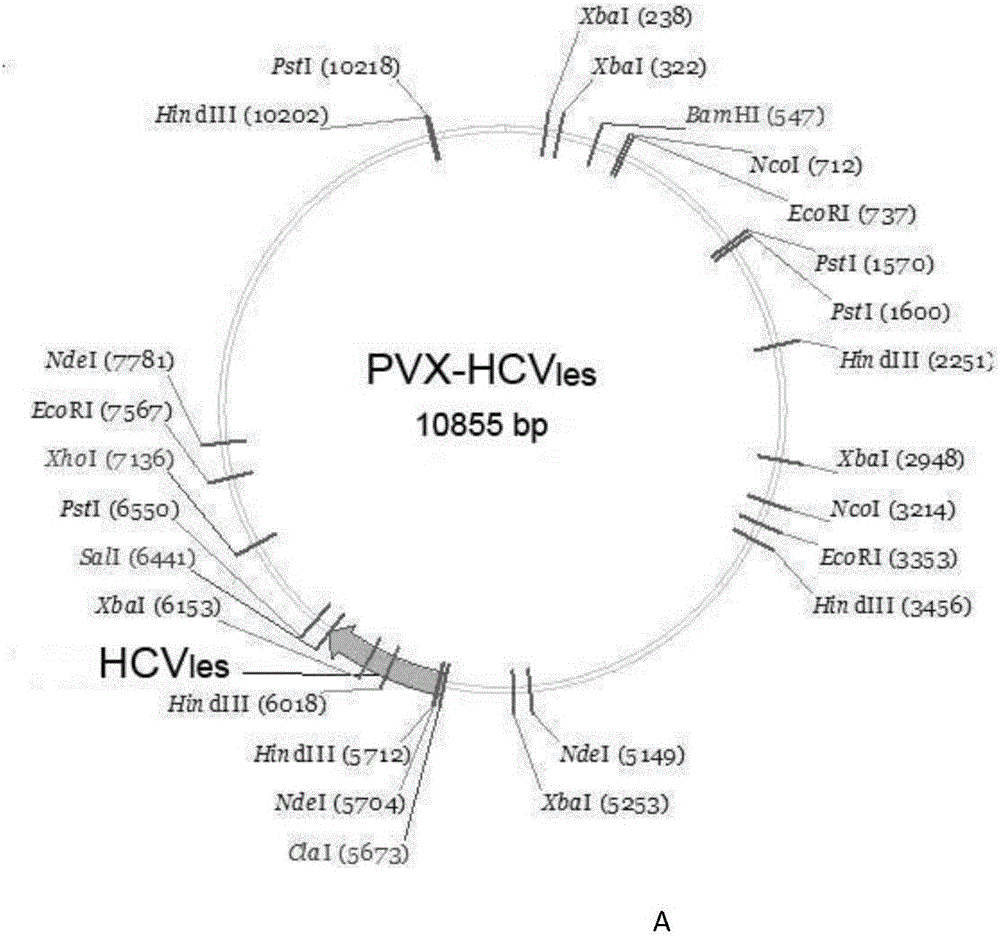
[0049] 2.1 Construction of fusion antigen HCVles expression vector
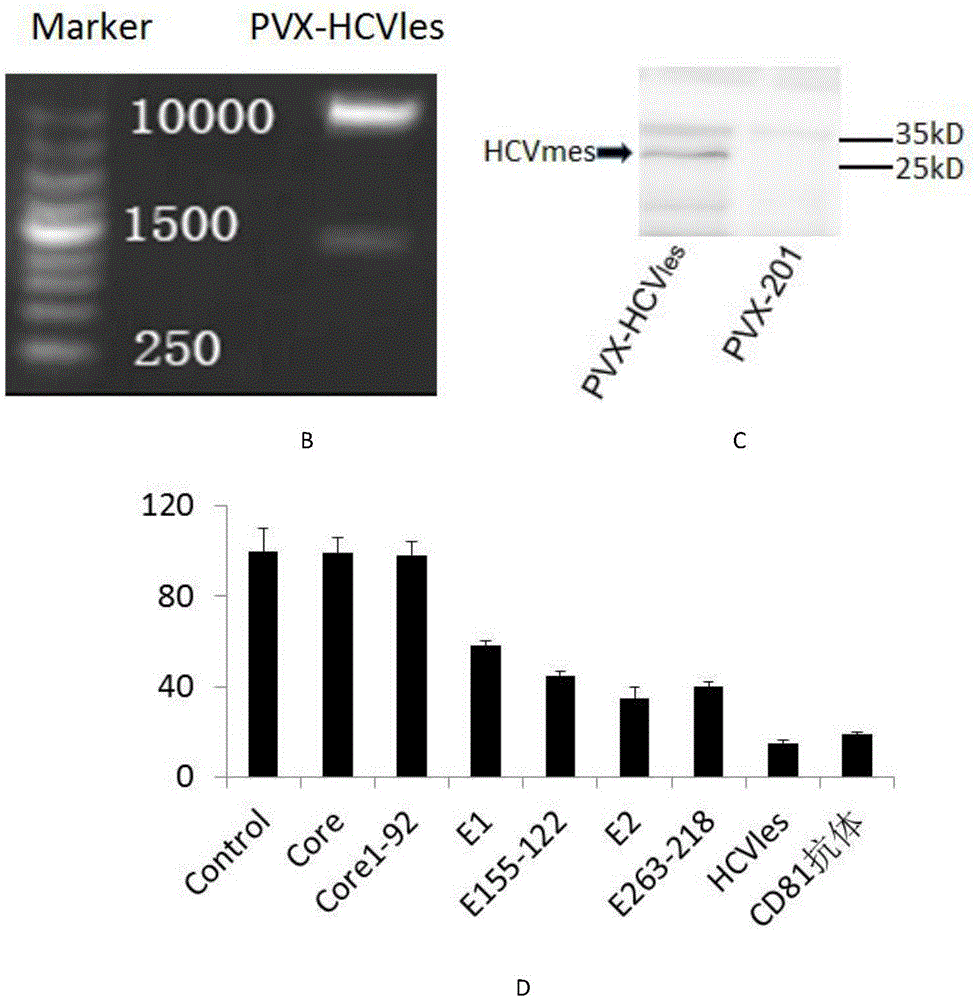
[0050] The fusion antigen HCVles gene was synthesized by overlapping PCR, and the HCVles gene encodes 316 amino acids (as shown in SEQ ID NO.1), which consists of the antigenic polypeptide Core with strong immunogenicity 1-92 , E1 55-122 and E2 63-218 Composed in series. To facilitate HCV les Gene cloning, protein expression and purification, in HCV les A ClaI restriction site and a tobacco-preferred Kozak sequence, a histidine tag (6 histidines) were introduced into the 5' end of the gene, and a stop codon and a SalI restriction site were introduced into the 3' end. 24 primers (SEQ ID NO.2-25) were designed, Sangon Bioengineering Co., Ltd. synthesized the above 24 primers, and a fusion protein gene (SEQ ID NO.26) was synthesized by one-step overlapping PCR reaction. T...
Embodiment 3
[0066] Example 3 fusion antigen Core 1-92 / E1 55-122 / E2 63-218 Determination of reactivity between (HCVles) and serum of hepatitis C patients
[0067] ELISA assay for HCVles, Core, Core 1-92 ,E1,E1 55-122 , E2 and E2 63-218 Reactivity with serum from patients with hepatitis C virus. With purified antigenic polypeptides (HCVles, Core, Core 1-92 ,E1,E1 55-122 , E2 and E2 63-218 ) was coated on a 96-well plate, and the amount of coating protein per well was 50ng. Human serum was the primary antibody, and the secondary antibody was human HRP-labeled secondary antibody (Wuhan Sanying Biotechnology Co., Ltd.). HCV-negative sera served as negative controls. HCV les Reacted with 98 out of 100 randomly selected sera of hepatitis C virus-infected patients (from Wuhan University People's Hospital, Ningbo People's Hospital and Nanchang Liver Disease Hospital), the response rate was 98%, which was significantly higher than Core, Core 1-92 ,E1,E1 55-122 , E2, and E2 63-218 T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com