Method for determining content of boron in boride
A kind of determination method, the technology of boron content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Determination of Boron Oxide Content in Boron Nitride
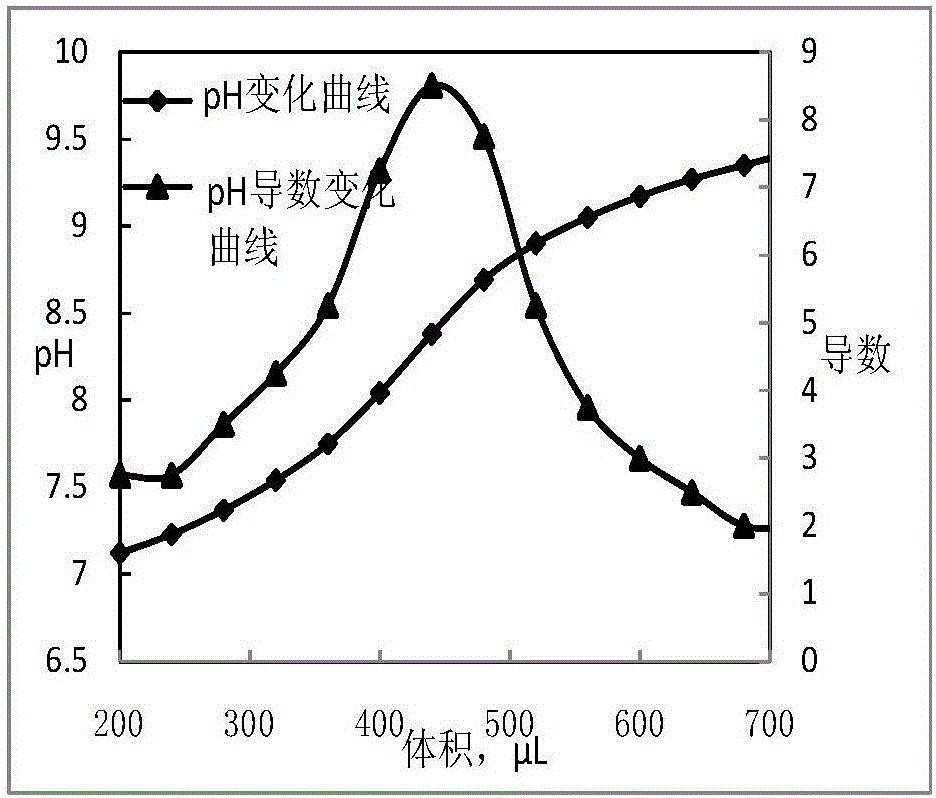
[0026] Weigh 1g of ERM-ED103 sample (accurate to 0.1mg), dissolve it in hot water at 70°C for 40min, then suction filter while it is hot, collect the filtrate in a 500mL Erlenmeyer flask, transfer it to a three-necked flask after cooling under running water, add 5g of mannitol, and add Rotor, titrate the reaction liquid with 0.05mol / L NaOH standard solution, use the pH standard solution to calibrate the pH meter, measure the pH change of the solution with the pH meter, and collect data every 30s. The titration curve takes the titration volume as the abscissa, and the pH value and The derivative is the ordinate, and the titration curve is derived, and the maximum point of the derivative is selected as the titration end point, and the corresponding consumed NaOH solution volume is the reaction end volume. The boron oxide content is calculated by formula (1).
[0027] Titrate with 0.05mol / L NaOH standard solution, th...
Embodiment 2
[0031] Determination of total boron content in boron nitride
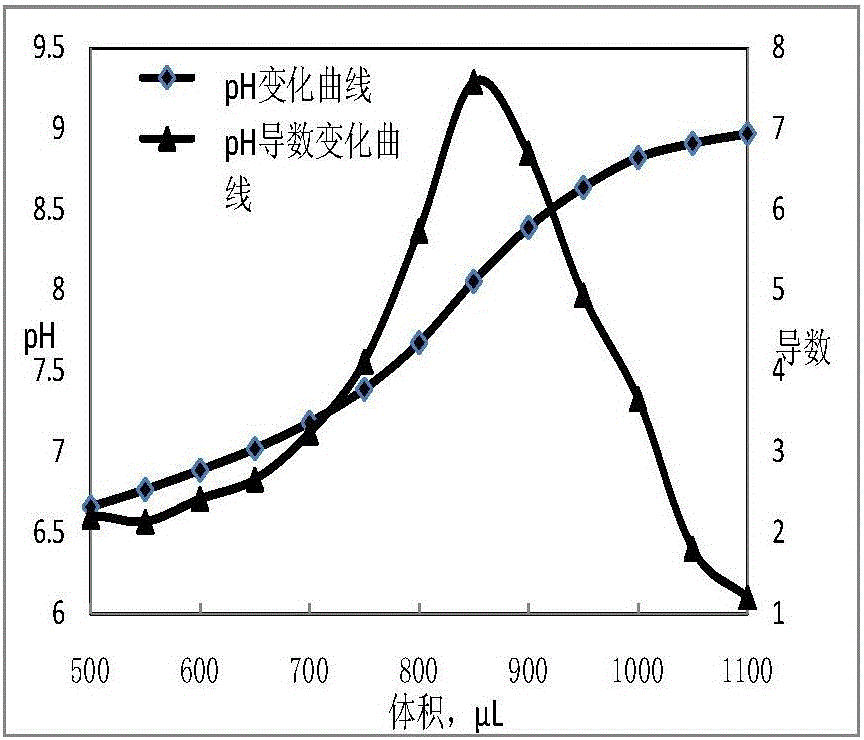
[0032] Weigh 0.1g (accurate to 0.1mg) of the ERM-ED103 sample into a nickel crucible, add 3g of NaOH solid, cover, slightly open the cover, put it into the muffle furnace, heat up to 700°C, melt for 10min, after cooling, use Lip the molten material in hot water into a 250mL beaker, add calcium carbonate in portions and keep stirring until no bubbles are generated, boil on an electric stove for 3 minutes, then filter with suction after cooling slightly, wash the flask with hot water 5 times, wash the precipitate 10 times, collect Add 1 drop of (1+1) hydrochloric acid and 1 drop of methyl red to the filtrate in a 500mL Erlenmeyer flask, boil for 5 minutes, transfer to a three-necked flask after cooling under running water, and titrate with 0.1mol / L NaOH solution until it turns from reddish to just Yellow (not counting the volume), add 5g mannitol, add the rotor, use pH standard solution to calibrate the acidity meter...
Embodiment 3
[0036] Determination of Boron Oxide in Boron Carbide
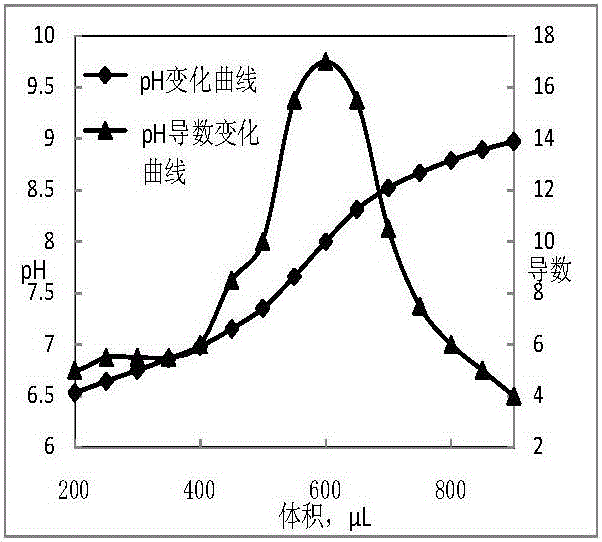
[0037] Weigh 0.5g (accurate to 0.1mg) of ERM-ED102 sample, dissolve it in hot water at 70°C for 40 minutes, then filter while hot, collect the filtrate in a 500mL Erlenmeyer flask, transfer it to a three-necked flask after cooling under running water, add 5g of mannitol, Add the rotor, use the pH standard solution to calibrate the acidity meter, use 0.02mol / L NaOH solution for titration, use the acidity meter to measure the pH change of the solution, and collect data every 30s. The titration curve takes the titration volume as the abscissa, and the pH value Its derivative is the ordinate, and the titration curve is derived, and the maximum point of the derivative is selected as the titration end point, and the corresponding consumed NaOH solution volume is the reaction end volume. The boron oxide content is calculated by formula (1).
[0038] Titrate with 0.02mol / L NaOH standard solution, the sampling pH time interval is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com