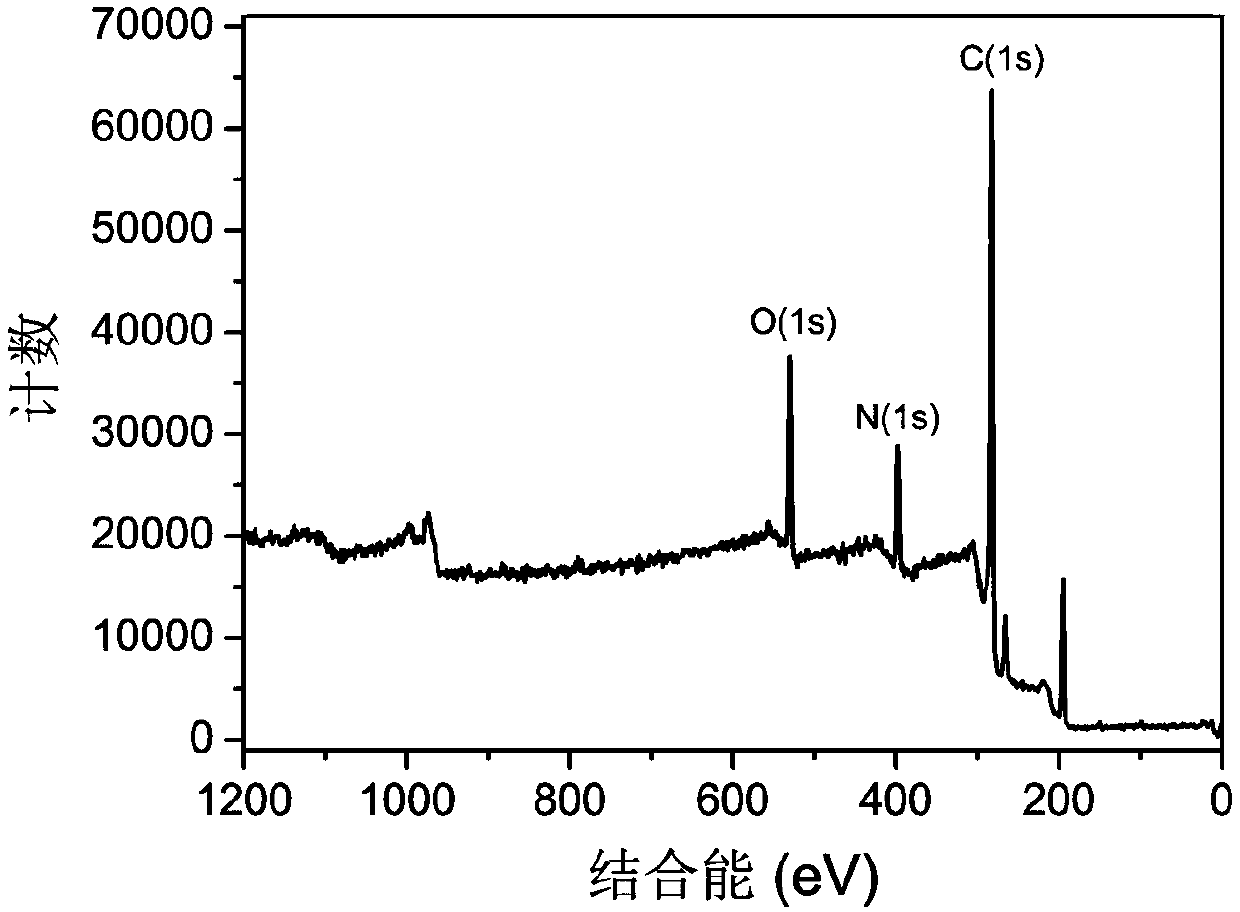
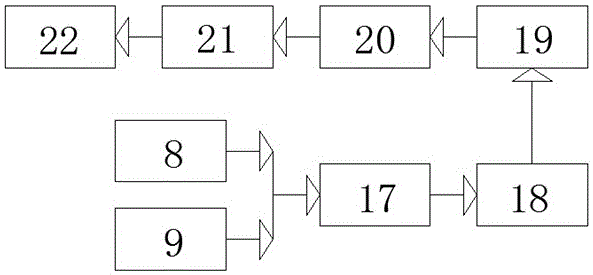
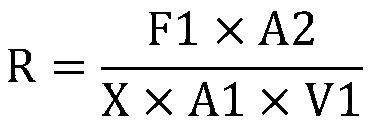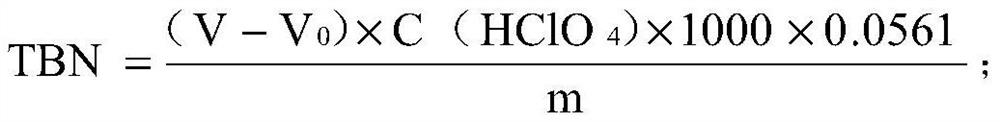Patents
Literature
52 results about "Titration curve" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
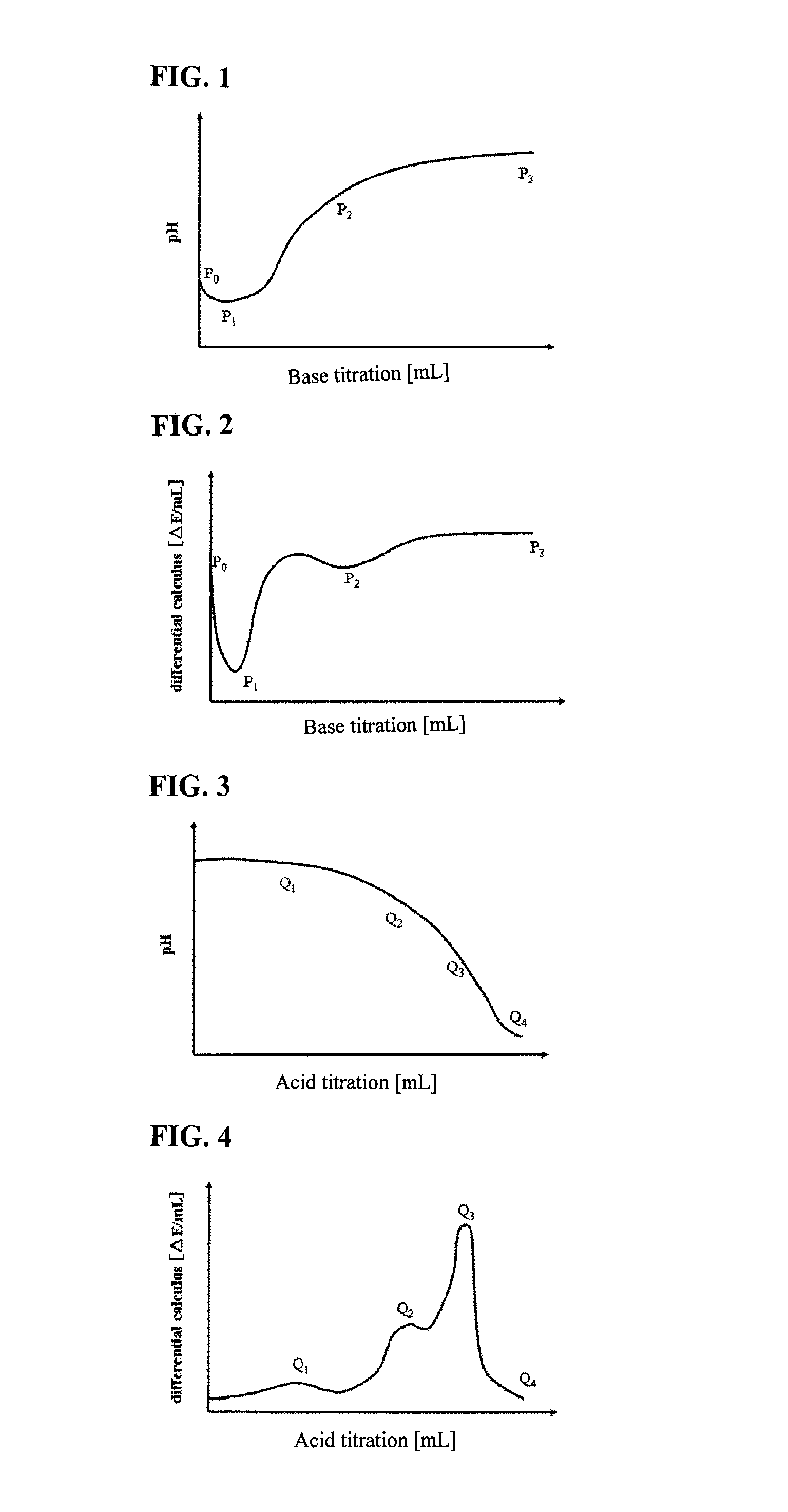
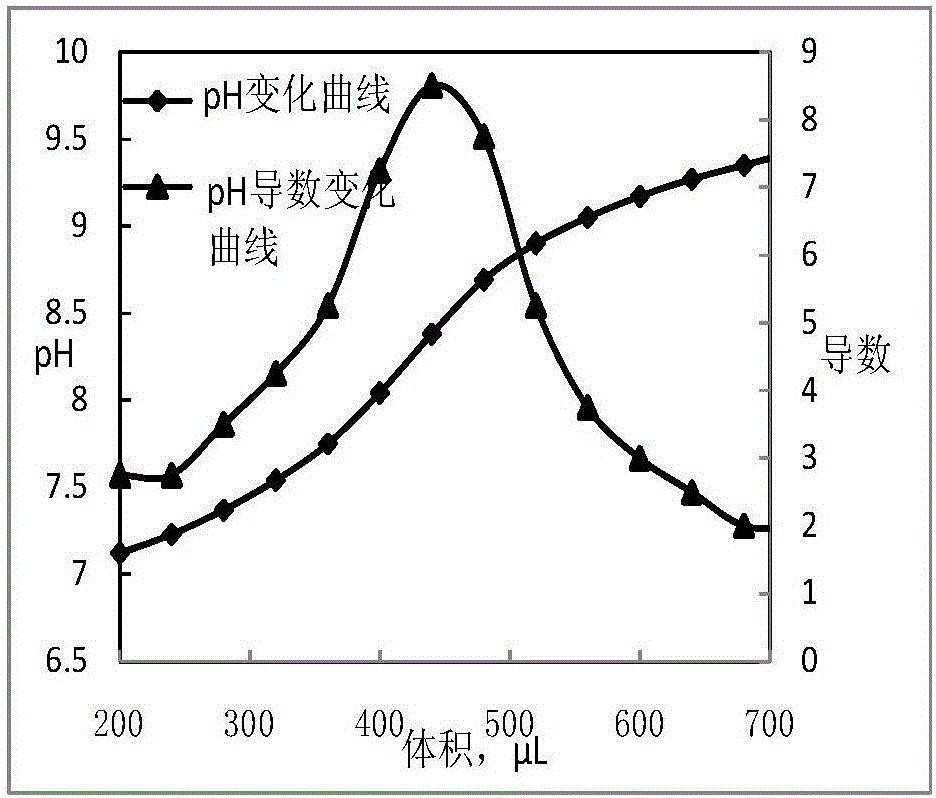
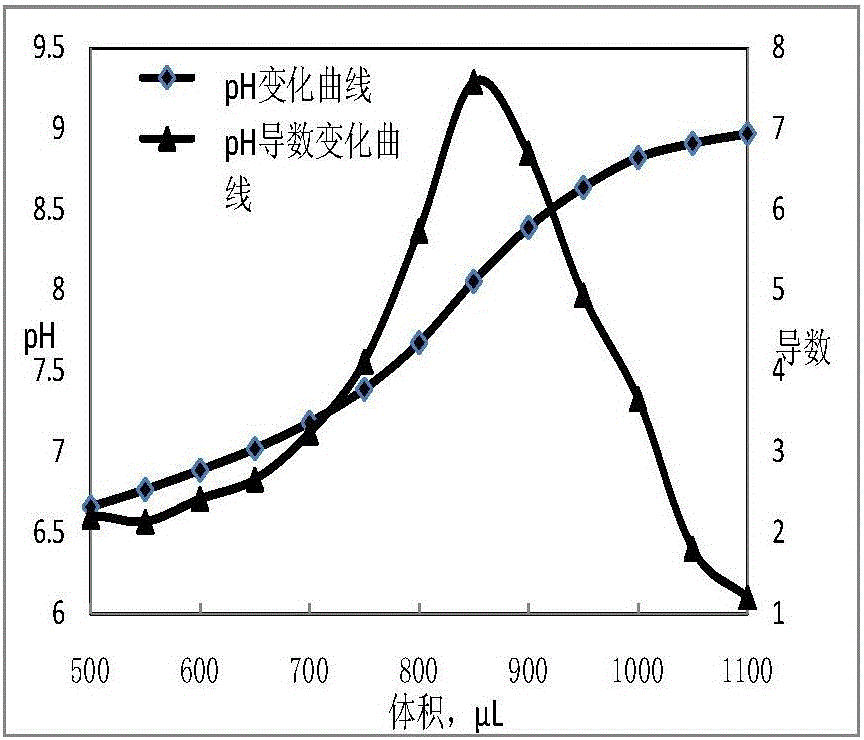
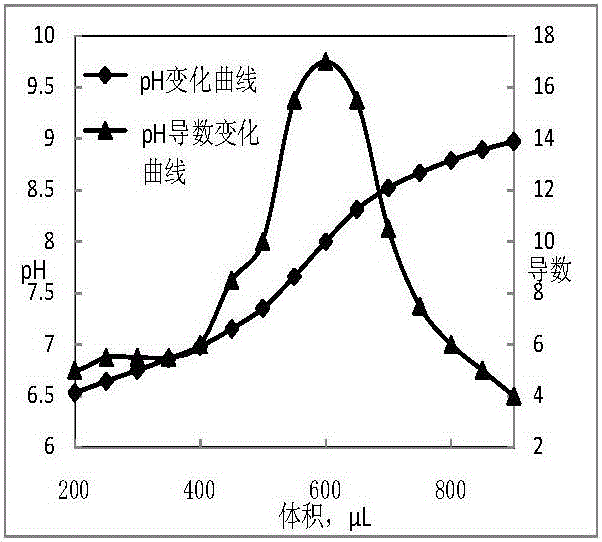
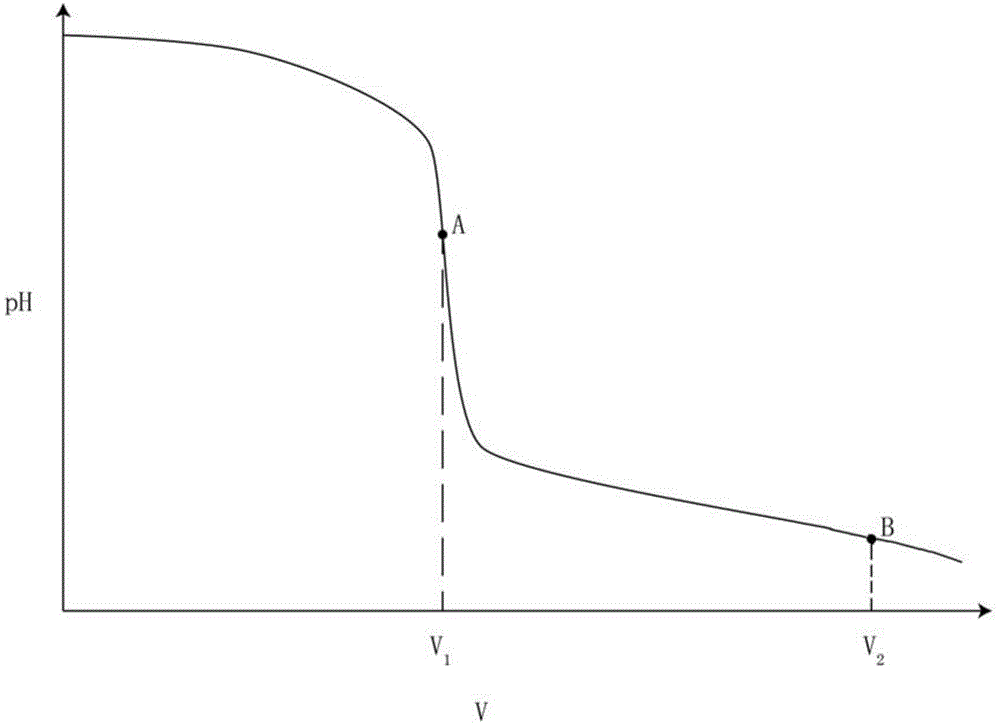
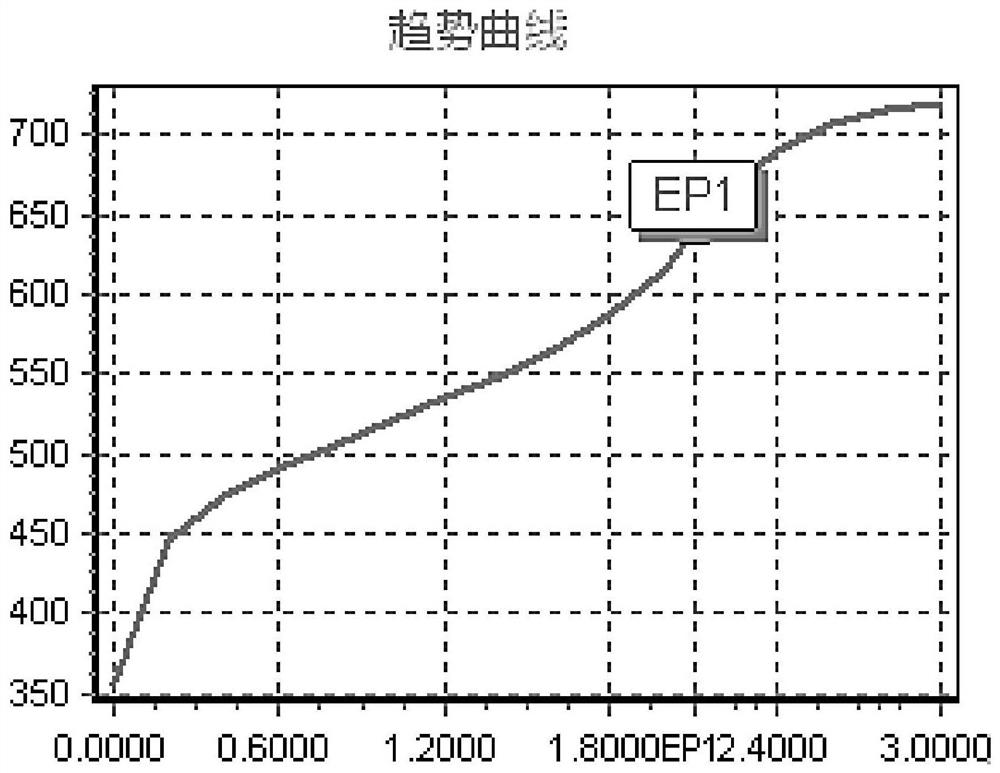
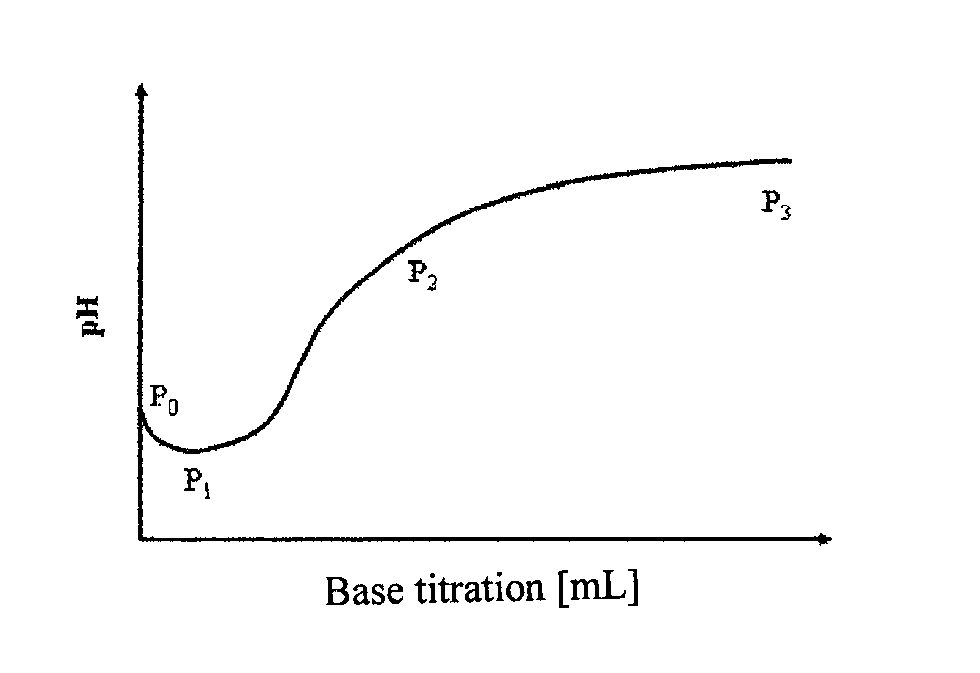
Titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the independent variable and the pH of the solution as the dependent variable (because it changes depending on the composition of the two solutions). The equivalence point on the graph is where all of the starting solution (usually an acid) has been neutralized by the titrant (usually a base).
Quantitatively analyzing method for fluohydric acid in lithium ion battery electrolyte
InactiveCN1614406AHigh measurement accuracyEasy to judgeChemical analysis using titrationMaterial electrochemical variablesHydrofluoric acidAlcohol
A quantitative analysis method includes diluting lithium ion cell electrolyte in absolute ethyl alcohol or methyl alcohol, using MOH as titrant, applying automatic potentiometric titration, using (CoXV20) / (1000XM) to confirm titrimetric curve as Co referring to tritrant concentration, V referring to consumed tritrant volume ml, 20 referring to HF molecular weight and M referring to electrolyte weight, using potentiometric titrator to carry out second order derivation of titrimetric curve for cnofirming titrimetric end point.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Quantum dot-encoded bead set for calibration and quantification of multiplexed assays, and methods for their use
InactiveUS20060131361A1Improve determinationImproved determinationCooking-vessel materialsNanoinformaticsAnalyteAssay
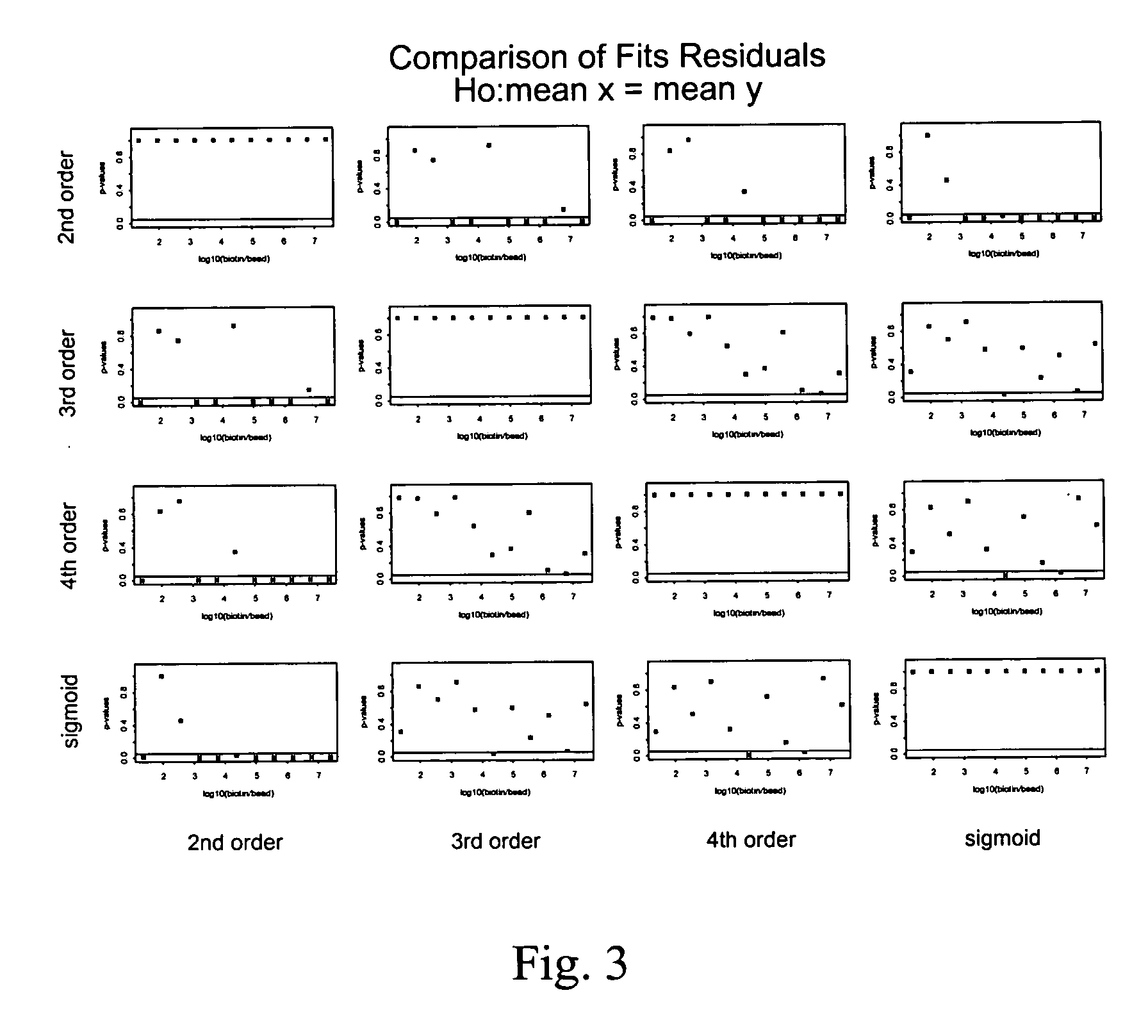
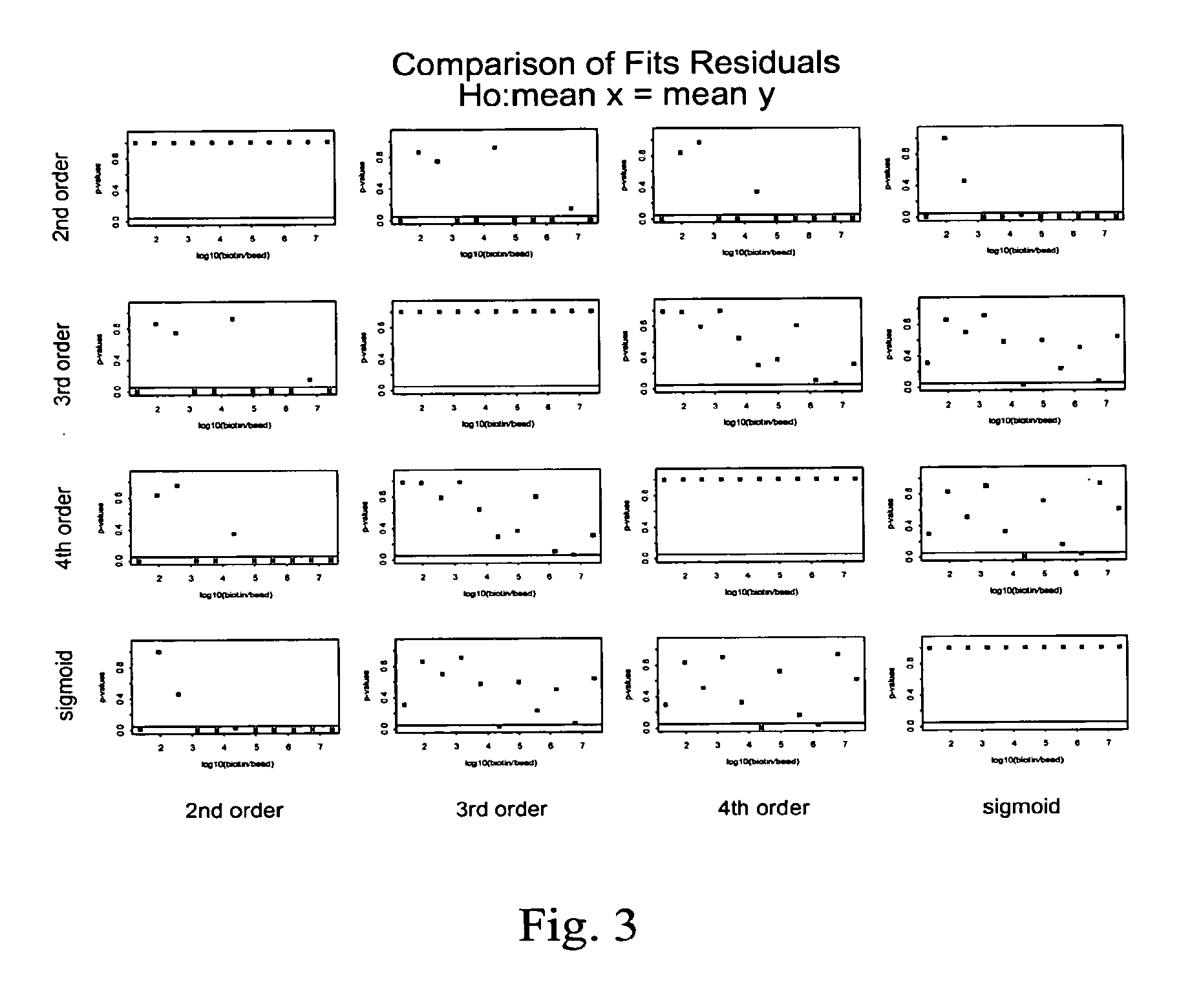
Control beads are disclosed that allow for improved quantitation of analytes in multiplexed bead assays. The control beads have a range of concentrations of calibration moieties that provide for the preparation of a titration curve. The titration curve can be used to quantify the concentration of the analytes. The titration curve can be used to correlate the signal obtained from a bead with the concentration (or absolute number of molecules) of the analyte bound to the bead.
Owner:LIFE TECH CORP
Automatic titration device for anaerobic fermentation buffer ability
ActiveCN104777267AAvoid manual titration human errorImprove work efficiencyChemical analysis using titrationVolatile fatty acidsData acquisition
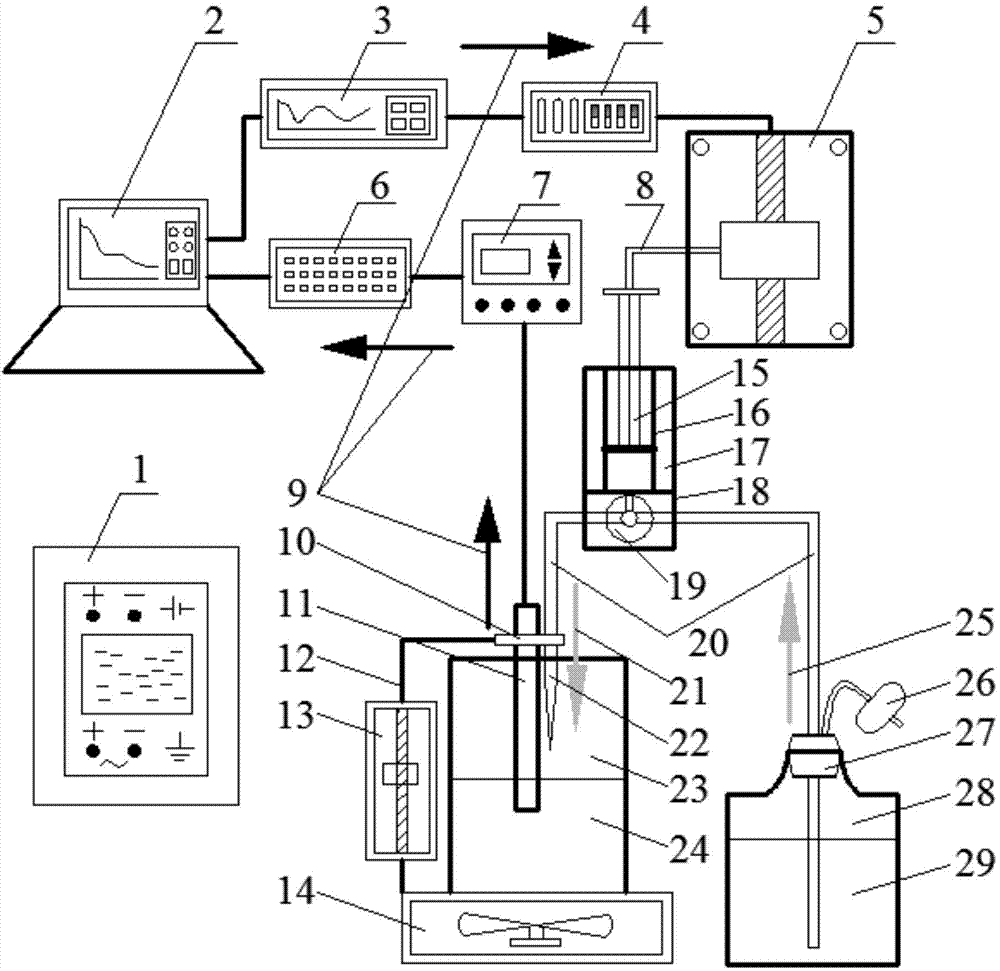
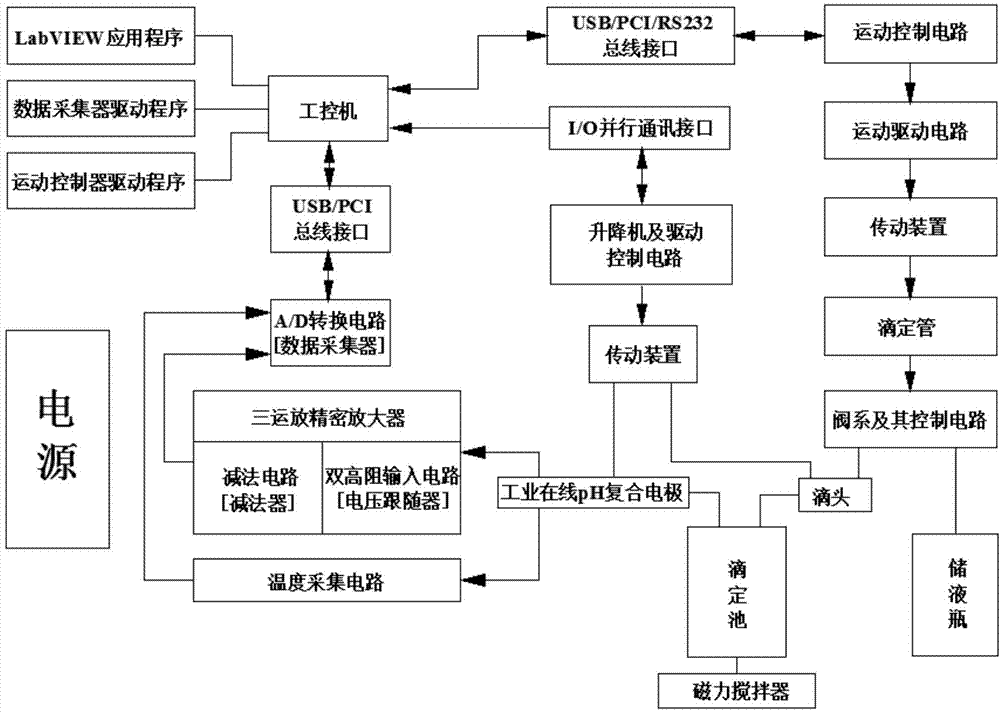
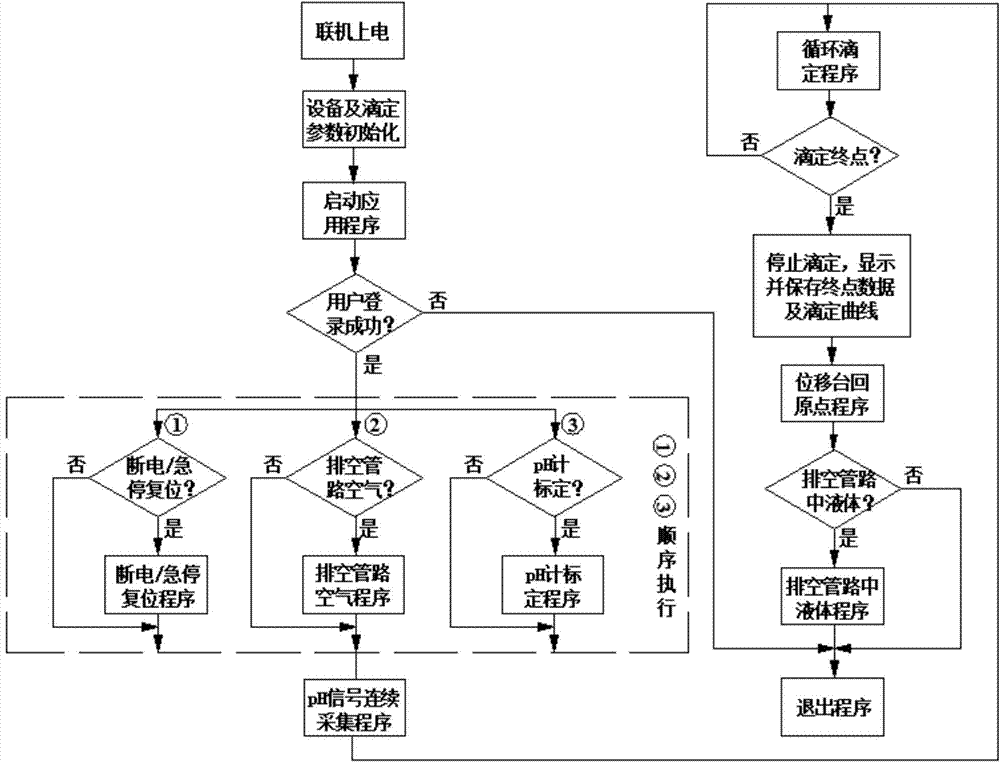
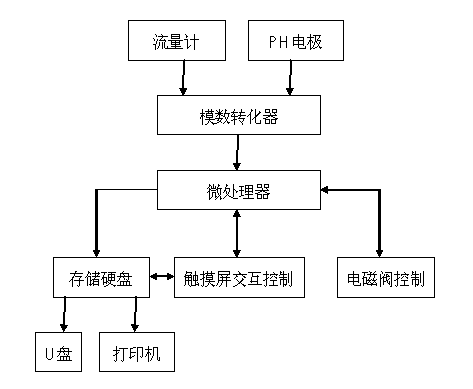
The invention discloses an automatic titration device for anaerobic fermentation buffer ability, which relates to the technical field of biological fermentation and is used for solving the problem of failure in simultaneous accurate measurement of mass concentration of total inorganic carbon (TIC) and volatile fatty acids (VFA) in anaerobic fermentation broth in the prior art. The automatic titration device comprises a power module, a man-machine exchange module, a data acquisition module, a driving transmission module and a liquid sucking / dropping module. The automatic titration device adopts the combined and integrated structure, application software provides a user operation panel, the panel of corresponding actual instrument realizes control and signal expression function of the instrument, the automatic titration device can simultaneously realize accurate measurement of mass concentration of TIC and VFA in the anaerobic fermentation broth by once titration, also can realize the functions of automatic calibration of a pH meter, automatic titration and liquid supplement, real-time generation of titration curve, data processing, display and storage, is suitable for pH, TIC and VFA mass concentration determination of anaerobic fermentation broth, and also can be used for titration of other samples.
Owner:CHINA AGRI UNIV
Aqueous dispersion type acrylic pressure-sensitive adhesive composition and pressure-sensitive adhesive tape
ActiveUS20110046296A1Emission reductionStrong adhesionAcid polymer adhesivesOrganic dyesPolymer scienceEmulsion
An aqueous dispersion-type acrylic pressure-sensitive adhesive composition includes acrylic copolymer emulsion particles dispersed in an aqueous medium. The acrylic copolymer emulsion particles has a ratio (ANIN) / (ANSUR) of 1 or more, wherein (ANIN) is an amount of acid groups in the acrylic copolymer emulsion particles, and (ANSUR) is an amount of acid groups on the surfaces of the acrylic copolymer emulsion particles, (ANIN) and (ANSUR) being calculated from a titration curve of potentiometric titration performed by adding an inorganic base solution to an acidic sample dispersion solution containing the acrylic copolymer emulsion particles dispersed in ion exchange water. A pressure-sensitive adhesive tape which strongly adheres to an adherend and has excellent removability can be formed using the aqueous dispersion-type acrylic pressure-sensitive adhesive composition.
Owner:DAINIPPON INK & CHEM INC
Weak acid mode pH sensitive type red light carbon quantum dot and preparation method thereof
ActiveCN107640759AWide response rangeEasy to operateNanoopticsNano-carbonFreeze-dryingTitration curve
The invention provides a weak acid mode pH sensitive type red light carbon quantum dot, and a preparation method and application thereof. The preparation method of the carbon quantum dot comprises thefollowing steps: 1) weighing p-phenylenediamine with certain mass, dissolving the p-phenylenediamine into secondary water, adding a small amount of concentrated HCl into the solution and performing ultrasonic treatment to obtain a uniformly mixed solution; 2) transferring the solution to a hydrothermal reaction kettle, standing after the reaction stops, cooling to room temperature, centrifuging to remove an undissolved substance, taking supernatant liquid, performing dialysis, and performing dialysis treatment in a glass container for at least three days to obtain the pure carbon quantum dotaqueous solution; and 3) freeze-drying the carbon quantum dot aqueous solution to obtain a purple powdered carbon quantum dot. In the carbon quantum dot, a pH titration curve takes on a double S model, so that the prepared carbon quantum dot has a double weak acid structural mode, contains two pieces of pKa, has wide response range on the pH value, and can be applied to preparation of a cell pH detection reagent.
Owner:SHANXI UNIV
Continuous potentiometric titration analysis method for micromolecule carboxylic acid and amino acid
InactiveCN102023197AOvercoming the problem of not being able to use weak jumps to judge titration measurement pointsTitration achievedChemical analysis using titrationStrong acidsPotentiometric titration
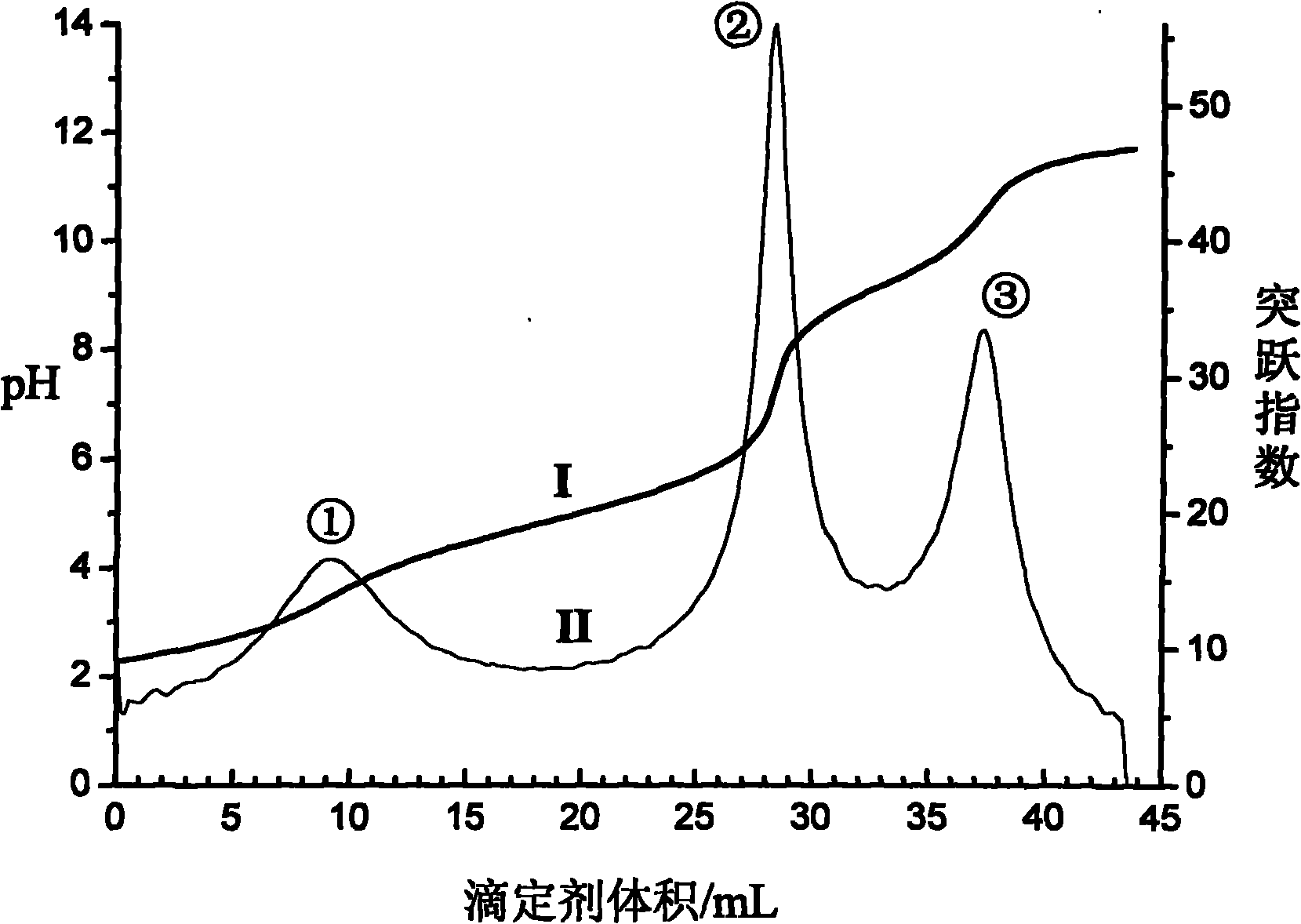
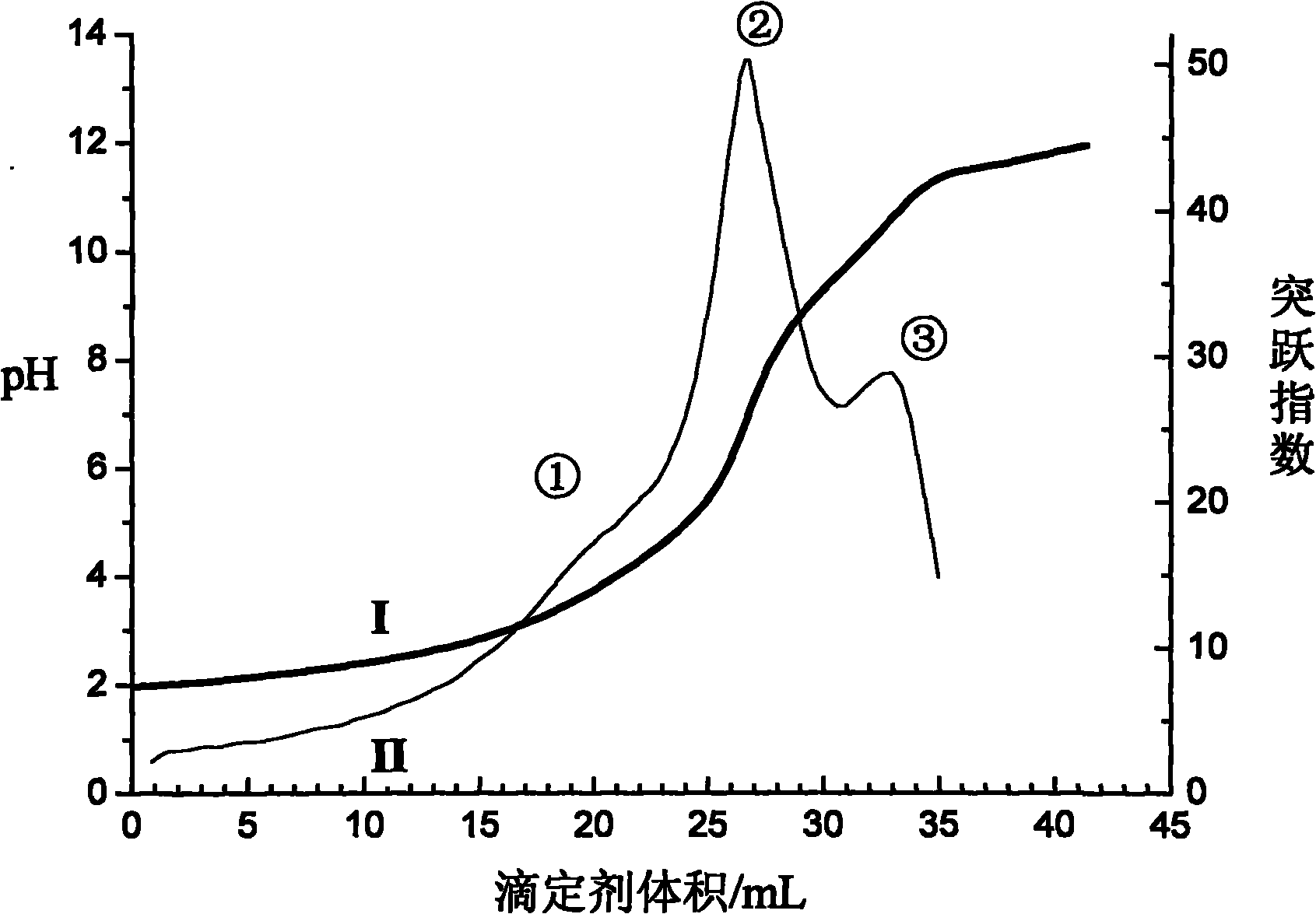
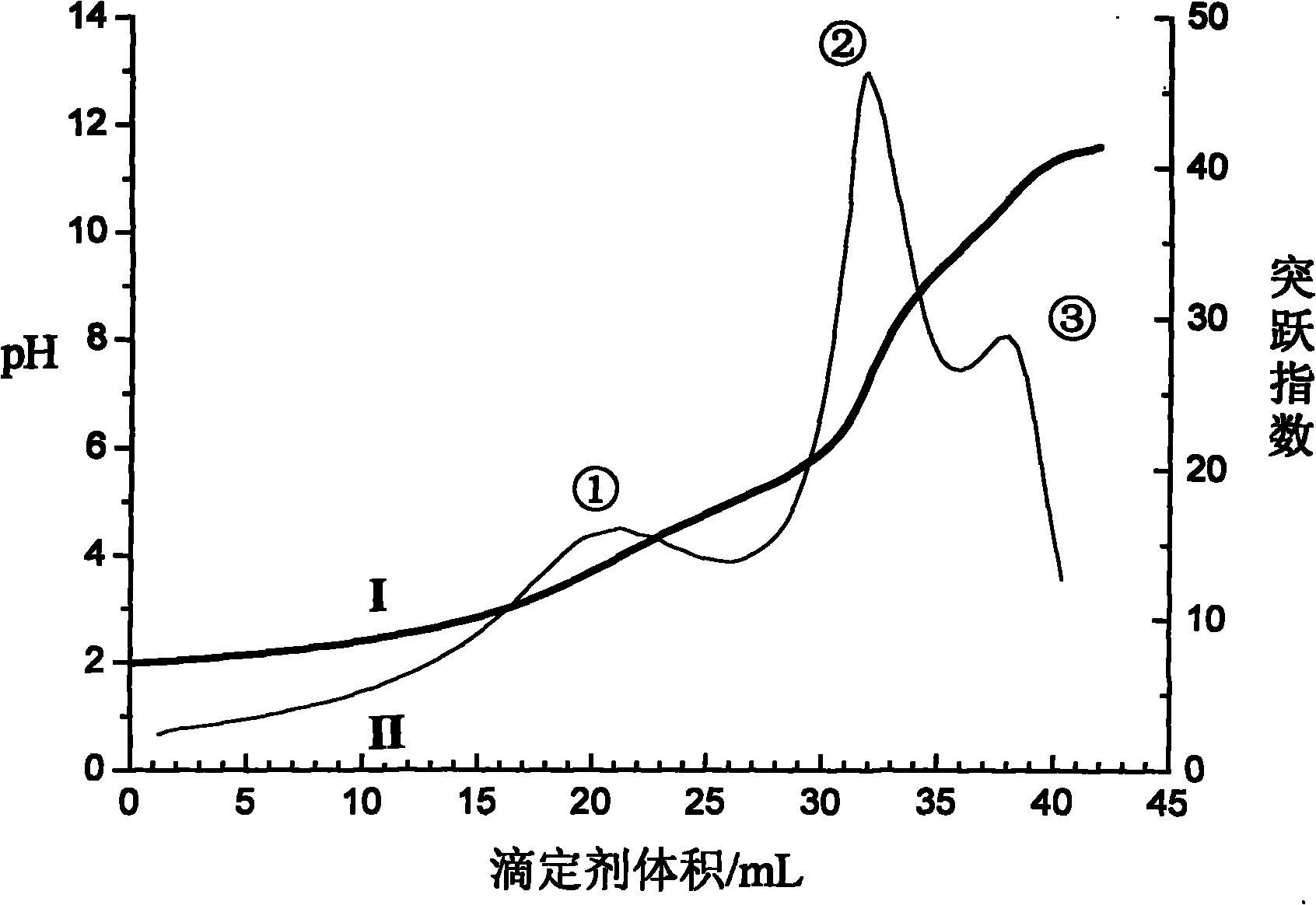
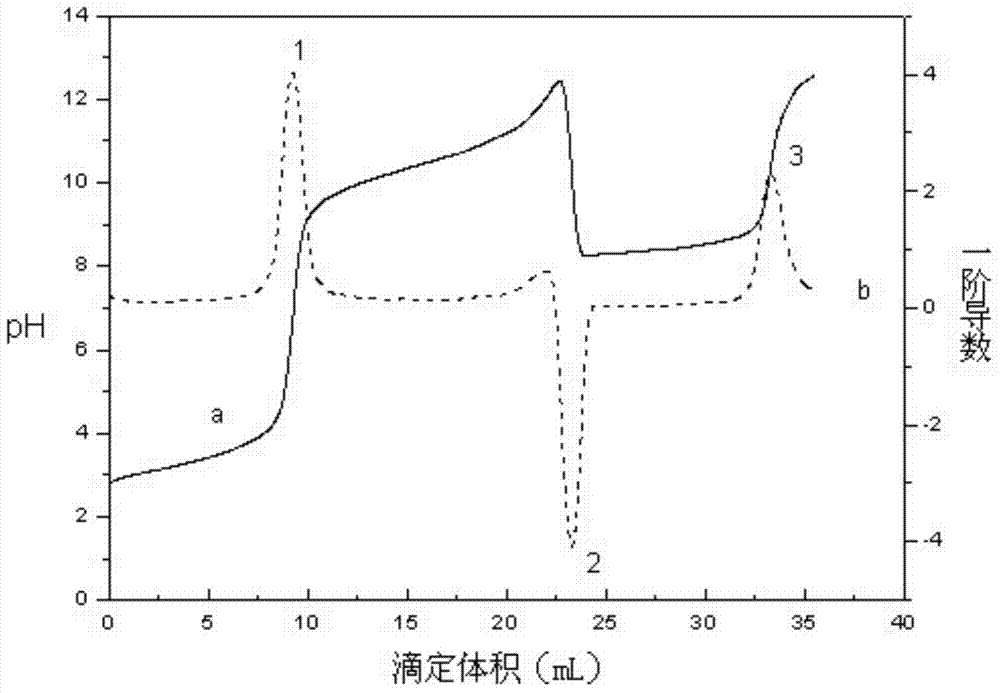
The invention discloses a volumetric analysis method, in particular to a continuous potentiometric titration analysis method for micromolecule carboxylic acid and amino acid in aqueous solution. The weak jump is enhanced by converting the first derivative of a titration curve into a jump index by a curve of the jump index to titrant volume; the pH value of the solution is regulated below 2 by using a strong acid when in titration; then the titration is carried out by using standard alkali liquor; a first jump is generated when the solution is titrated to have the pH value of 3-3.5, which shows that the free strong acid is titrated; the titration continues until the pH value of carboxyl is 7.5-9.3 to generate a second jump; then the titration continues until the pH value of amino is 11-11.5 to generate a third jump; and the consumed standard alkali liquor between the two adjacent jumps respectively reflect the total amount of the free strong acid, the carboxylic acid and the amino acid. The method is simple and convenient to operate and rapid to determine and has reliable result and low analysis cost, can be popularized and applied to analyzing other complicated samples.
Owner:GUANGXI UNIV
Automatic potentiometric titration capable of automatically judging terminal point
InactiveCN1392409AFast titration analysisHigh precisionMaterial electrochemical variablesPotentiometric titrationTitration curve
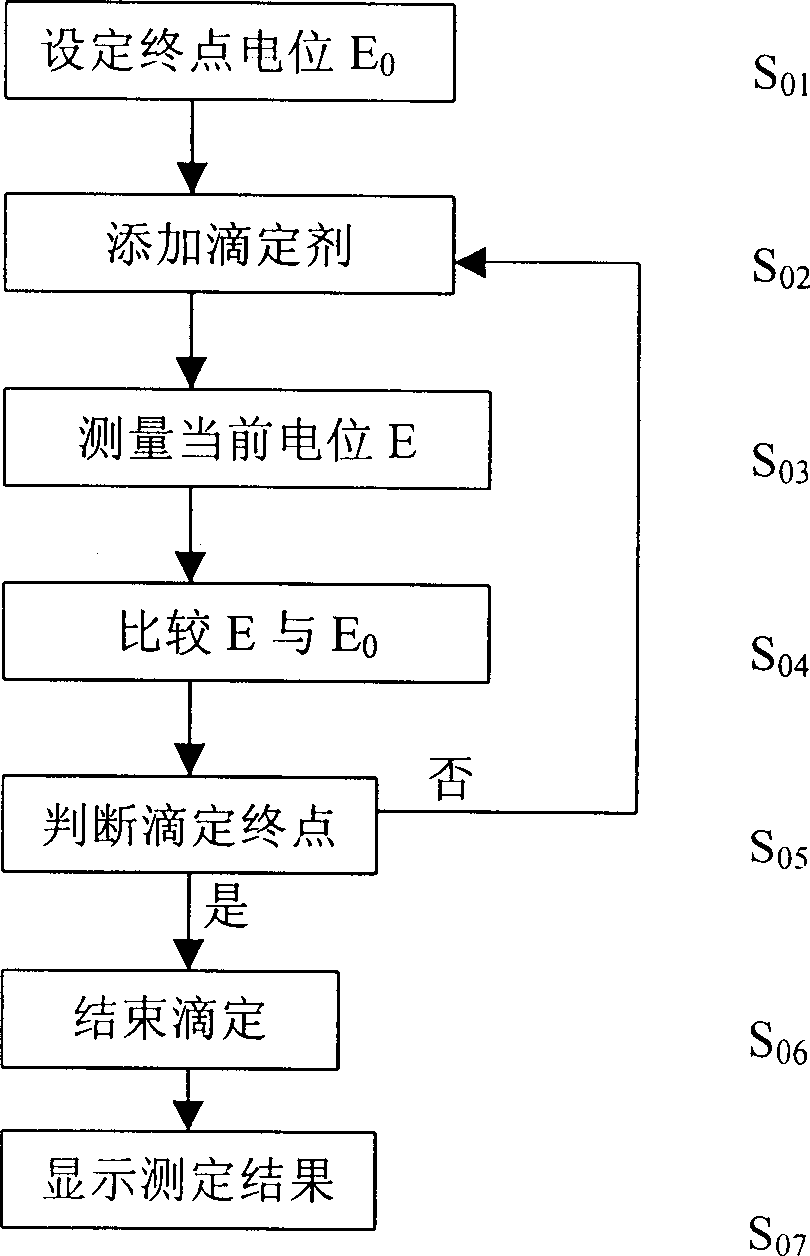
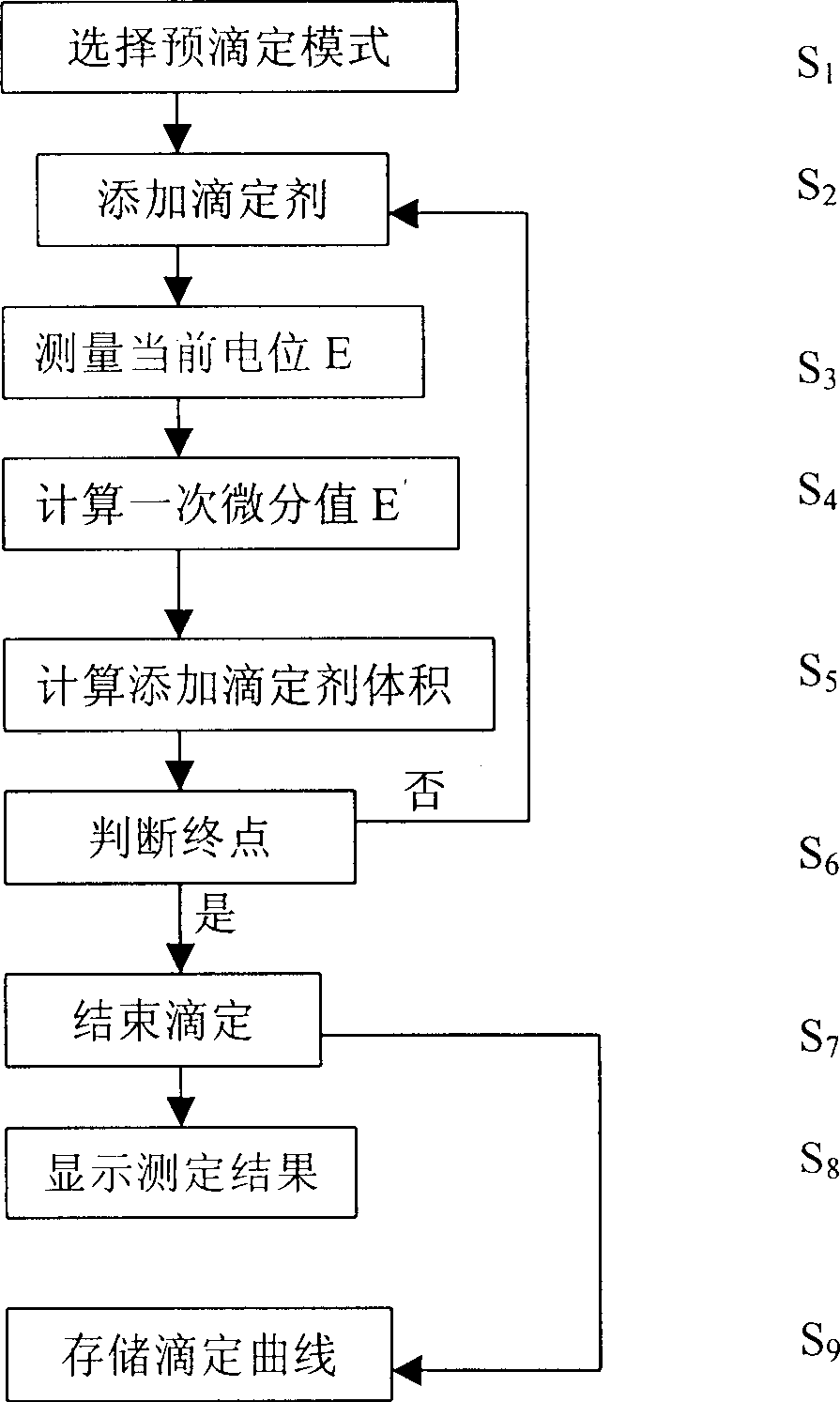
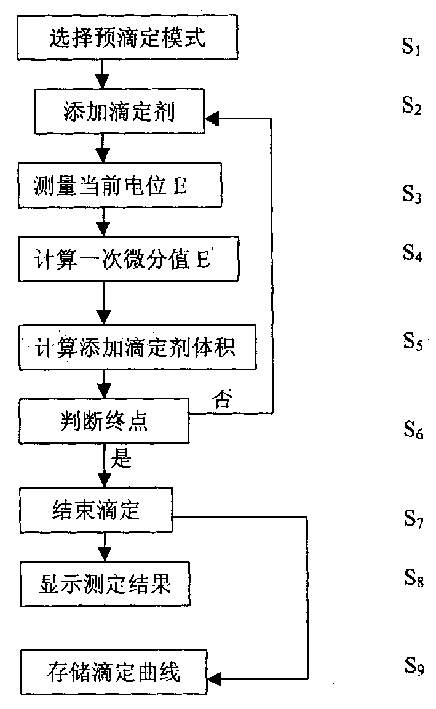
The present invention relates to electrochemical analysis technology and especially one automatic potentiometric titration capable of automatically judging end point. The titration process includes selecting titration mode, adding titrating agent, measuring present potential, calculating first order differential value, calcualting the volume of added titrating agent, judging the end point, endingthe titration, displaying the titration results and storing the titration curve. Meanwhile, the titration mode is selected in the computer, and the titration curve may be used as the titration mode for next similar titration analysis. The present invention has the advantages of fast titration speed, high precision, automatic titration process and simple operation process.
Owner:SHANGHAI PRECISION SCI INSTR
Method for determining mineral oil total acid value
InactiveCN101206192AReduce harmReduce pollutionSpecial data processing applicationsMaterial electrochemical variablesPotentiometric titrationAcid value
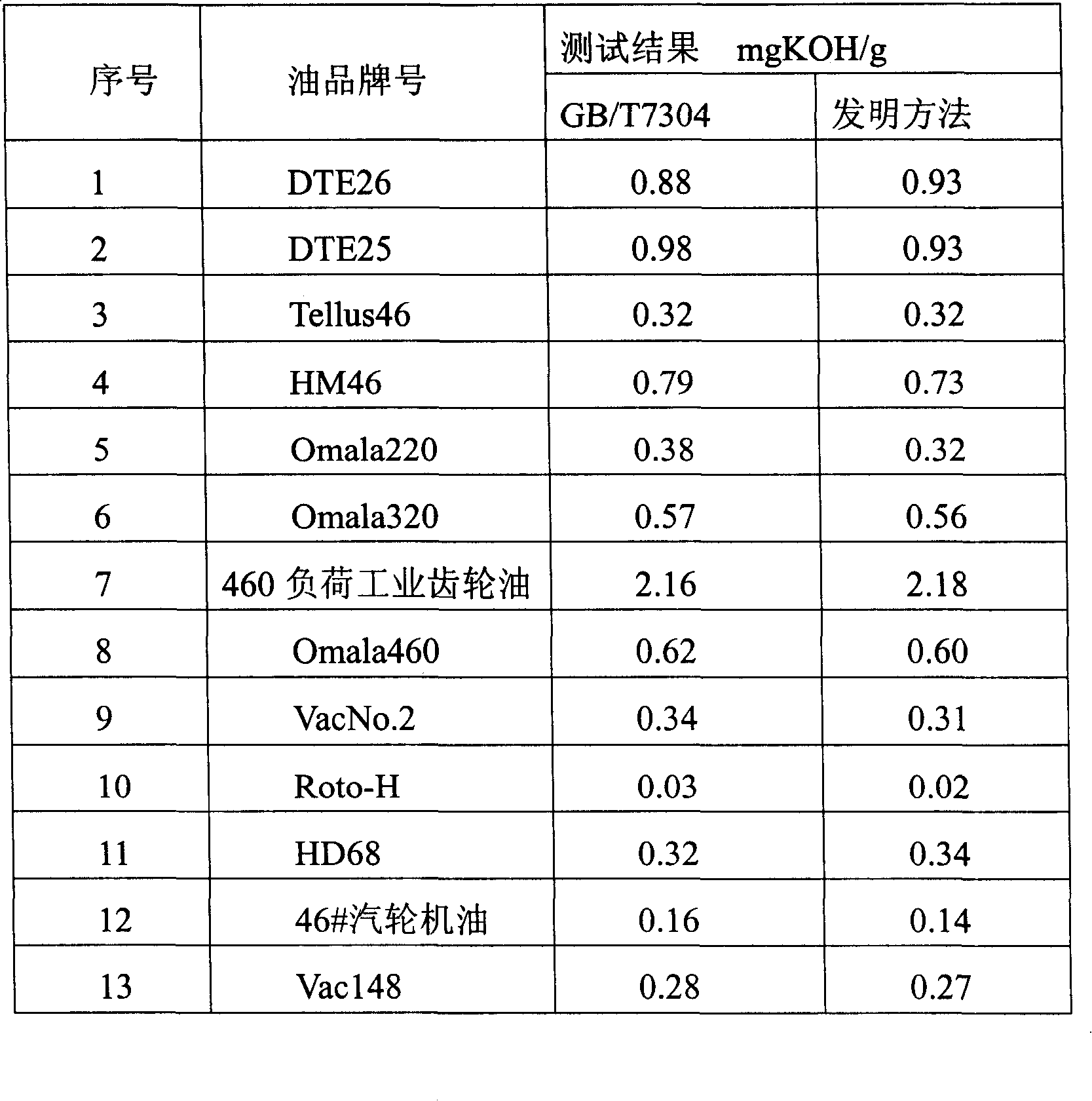
The invention relates to a measuring method for mineral oil acid value, implemented according to the following steps that: (1) petroleum ether and 99 percent ethanol aqueous solution are mixed according to the volume ratio of 1:1 to obtain mixed solvent; (2) KOH ethanol solution with the concentration being 0.05mol / L is prepared as volumetric solution; (3) according to the method of GB / T7304, the mixed solvent obtained in step (1) is measured by a potentiometric titrator to obtain a blank value; (4) a certain quantity of mineral oil sample, which is weighed out and is added in the mixed solvent, is stirred and heated to 45 to 55 DEG C, thereby ensuring that the mineral oil sample is completely dissolved in the mixed solvent; then, the mixture is cooled down; (5) according to the method of GB / T7304, 0.05molKOH / ethanol solution is used as volumetric solution to complete potentiometric titration on the potentiometric titrator; therefore, an end point can be judged by means of the potential jump on the potentiometric titration curve; finally, according to the volume of consumed volumetric solution, the total acid value of lubricating oil sample can be obtained through calculation.
Owner:SHANGHAI BAOSTEEL IND TECHNOLOGICAL SERVICE
Method and device for automatic determination of lime activity by titration
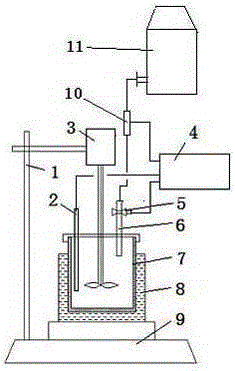
ActiveCN102798694AAutomate operationRealize instant controlChemical analysis using titrationWater bathsBurette
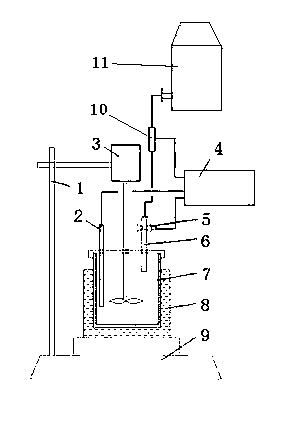
The invention relates to a method and a device for automatic determination of lime activity by titration, and the device comprises a titration rack, a burette, a flowmeter, a titration cup, a stirrer, a PH electrode, a titration control device, a titration table, and a water-bath heater. The burette is connected with a titrant bottle, and is provided with a titration switch. The titration control device is respectively connected with the PH electrode, the flowmeter and the titration switch circuit. The determination comprises the following steps: (1) accurately weighing 50.0 g of samples with a particle size of 1-5 mm, adding 4 mol / L hydrochloric acid into the titrant bottle; (2) setting the titration control device; (3) measuring 2000 ml water into the titration cup, stirring and heating to 40 DEG C; (4) adding the sample into the titration cup for digestion, starting the device for automatic titration; (5) displaying the consumption amount of the hydrochloric acid consumed by the titration. According to the invention, the PH value is used for titration control; the digestion reaction and the neutralization reaction are completed synchronously; precision of the hydrochloric acid amount is realized; the accuracy for lime activity determination is improved; and multifunctional analysis of the test data is realized through the titration control device.
Owner:SHIJIAZHUANG XINHUA IND FURNACE CO LTD
Automatic acid-base titration instrument
InactiveCN104730202AGuaranteed accuracyEasy to operateChemical analysis using titrationBuretteEngineering
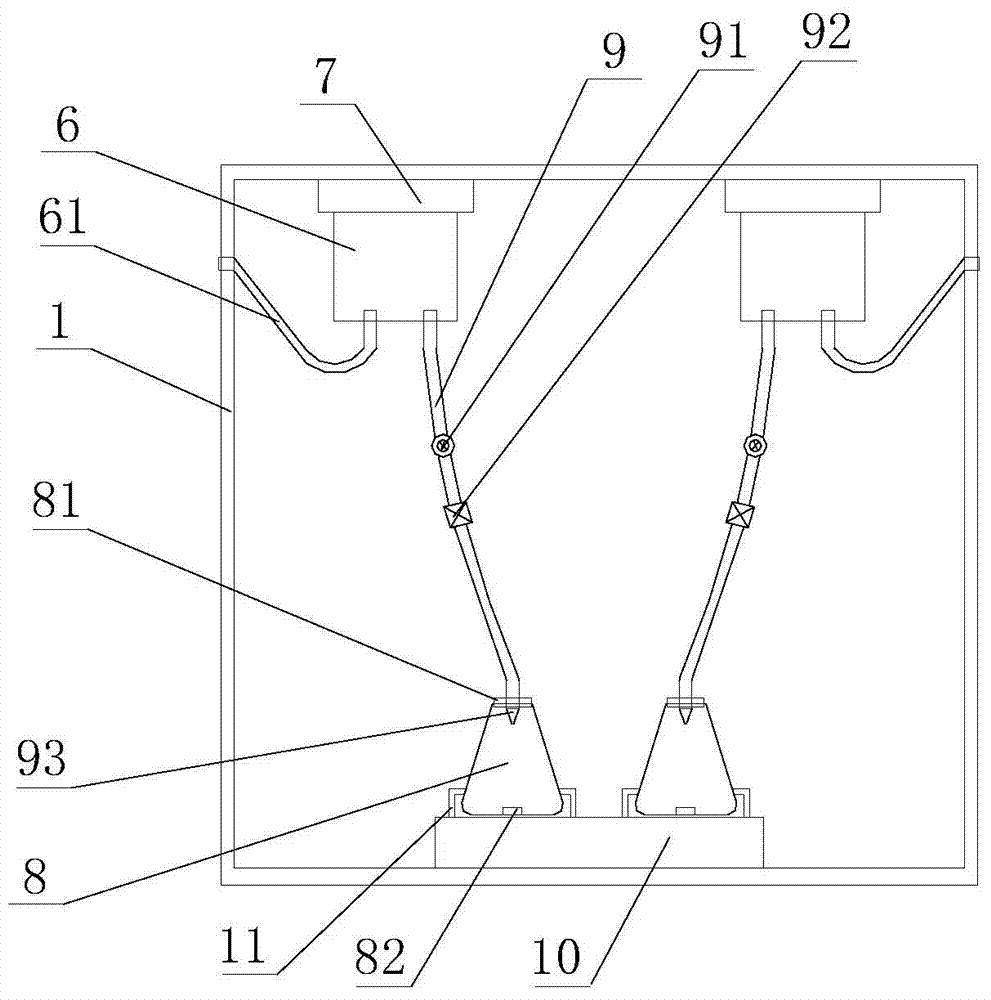
The invention relates to the technical field of experimental instruments, in particular to an automatic acid-base titration instrument, comprising a storage bottle, a titration tube, a vessel, a shell and an intelligent controller. The storage bottle is used for storing acid / alkali liquid; the vessel is used for storing liquid to be titrated; the shell is in a box structure. The storage bottle is fixed the top of an inner chamber of the shell through a fixing base. A vibrator is disposed within the inner chamber of the shell. The vessel is locked and fixed to the vibrator through a fixing clamp. The top end of the titration tube is connected to a liquid outlet of the storage bottle. The bottom end of the titration tube extends into the vessel. An orifice of the bottom end of the titration tube is connected with a sharp-tip titration tube. A micro-metering pump and an automatic valve are mounted on the titration tube in order from top to bottom. A pH sensor is disposed at the bottom of the inner chamber of the vessel. The automatic acid-base titration instrument is convenient to operate, safe and reliable, and time saving and labor saving, and accuracy of experimental results can be ensured.
Owner:HEZE UNIV
Method for determining amino value by automatic potentiometric titration
InactiveCN110726803AAvoid Difficult QuestionsSolve the problem that the jump is not obviousChemical analysis using titrationPotentiometric titrationTitration curve
The invention discloses a method for determining an amino value by automatic potentiometric titration, wherein by using the principle of a diazotization reaction between an aromatic primary amine compound and nitrite, taking a sodium nitrite standard solution as a titrant and a Pt electrode as an indicator electrode, and determining the titration end point by the maximum value of the first derivative of the titration curve, the quantitative analysis of the amino value is realized. The introduction of hydrobromic acid in the system and the control of the titration temperature solve the problemthat the potential titration end points of some low-active aromatic primary amine compounds do not jump significantly, enable control of the rate of the titration reaction, and can obtain test data more quickly and accurately. The method provided by the invention is convenient in operation, simple and fast, and reliable in results.
Owner:JIANGSU YANGNONG CHEM GROUP +2
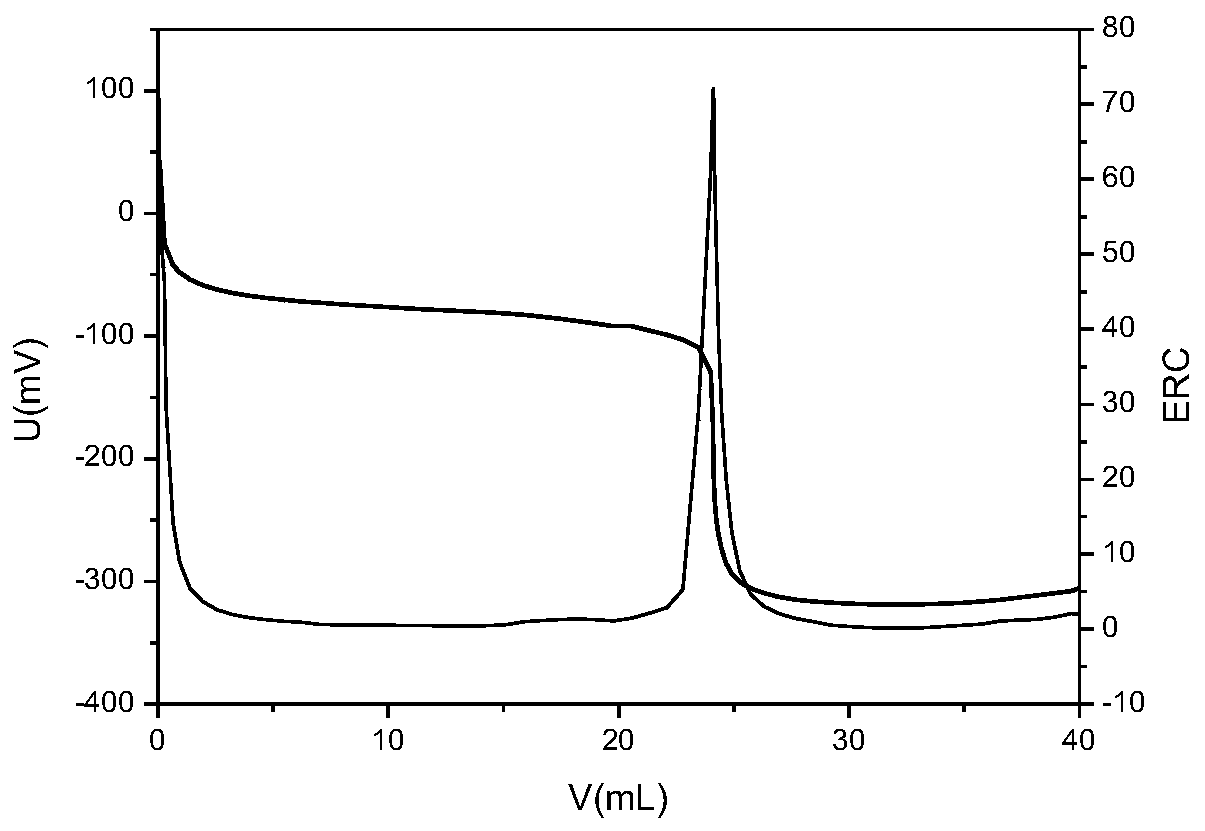
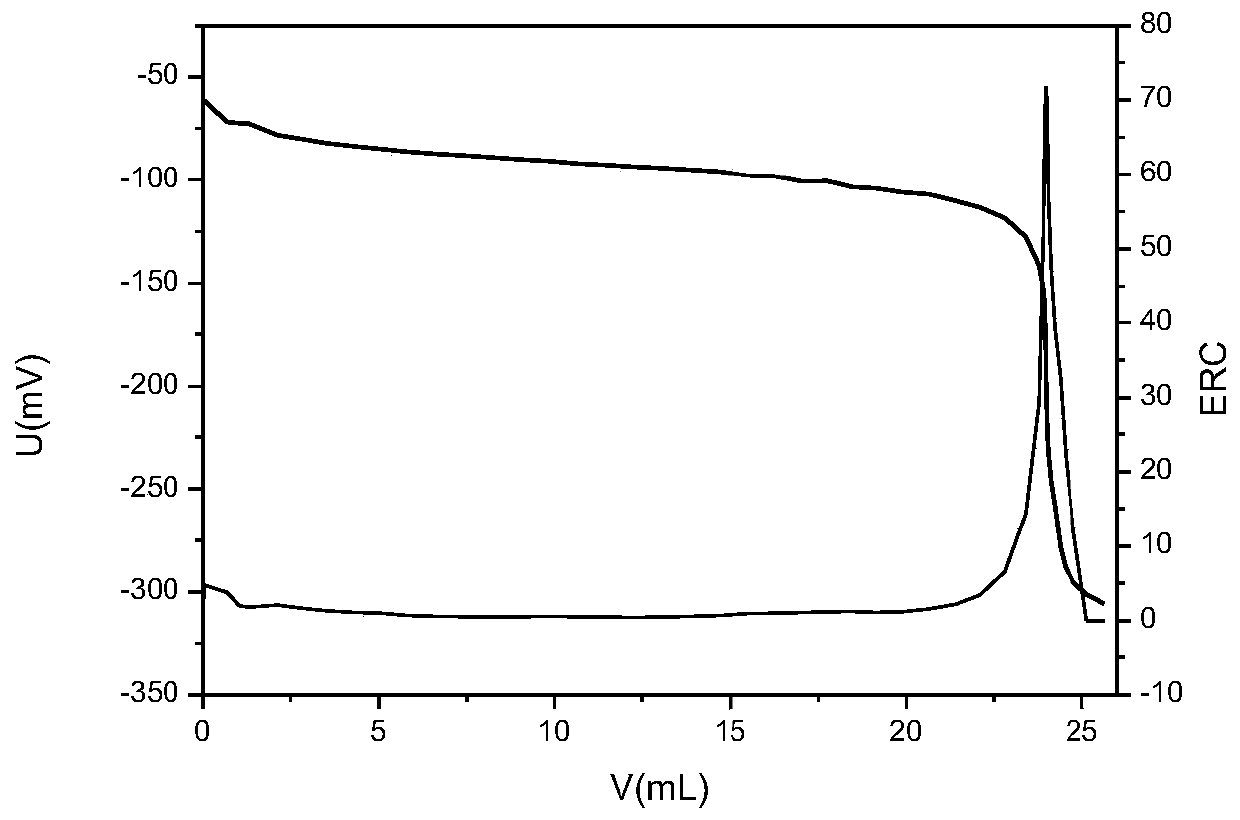
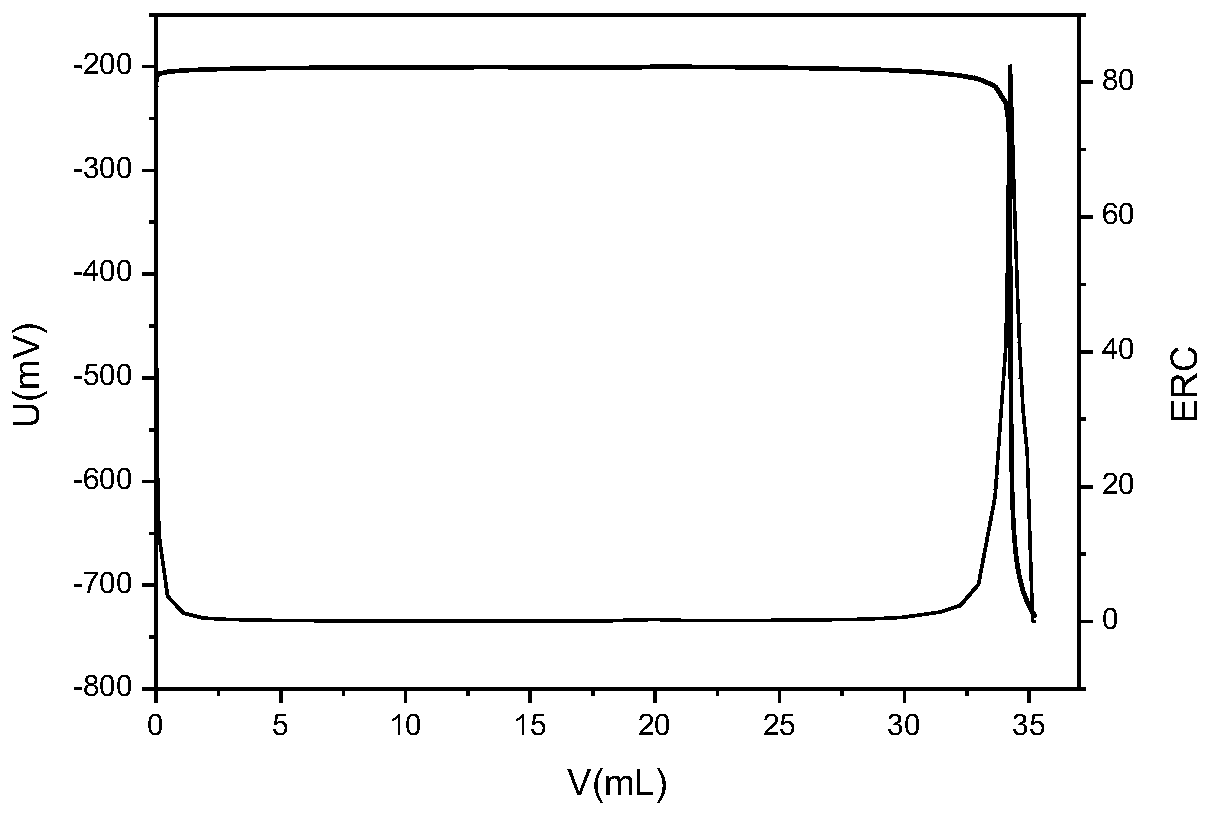
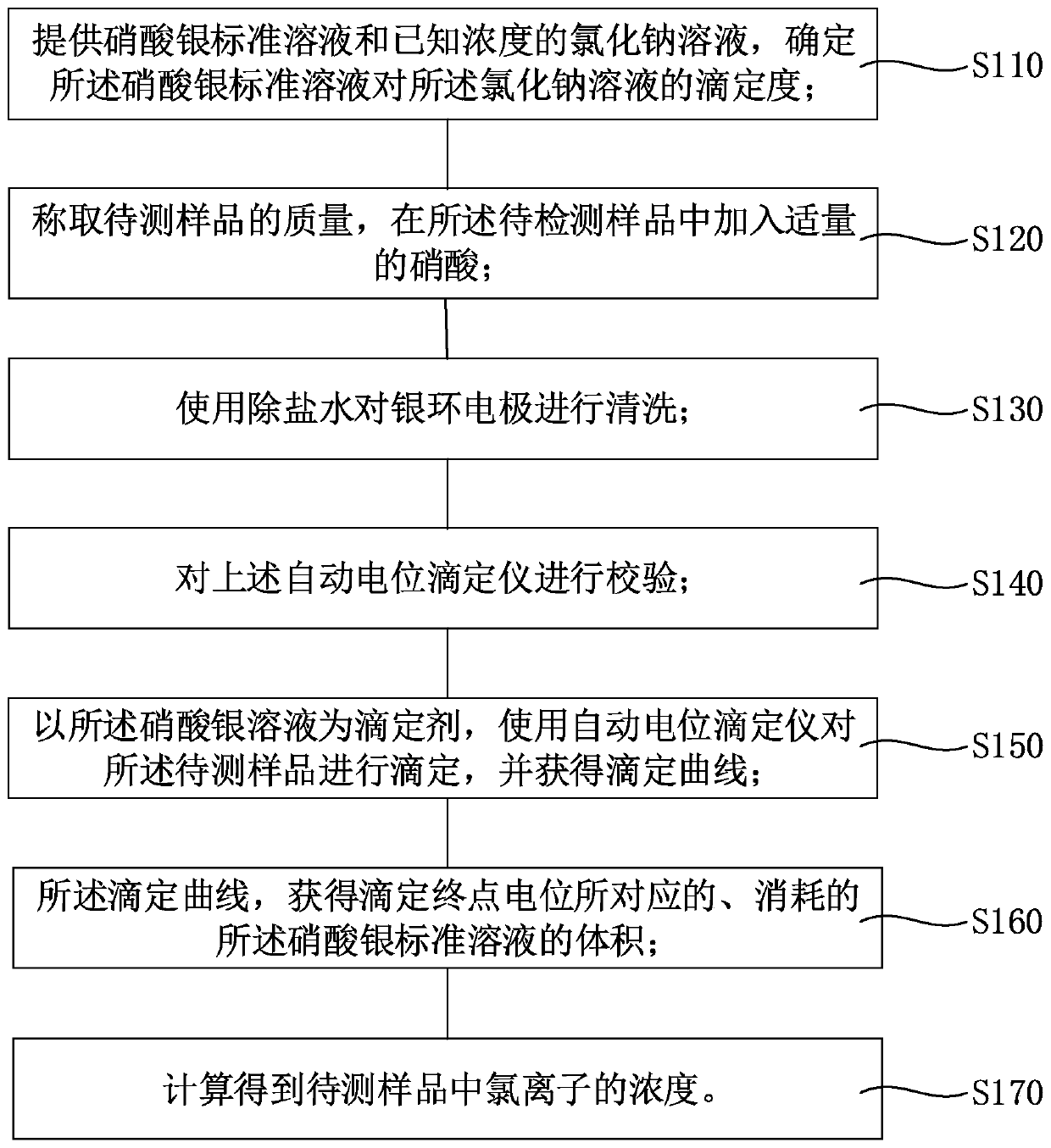
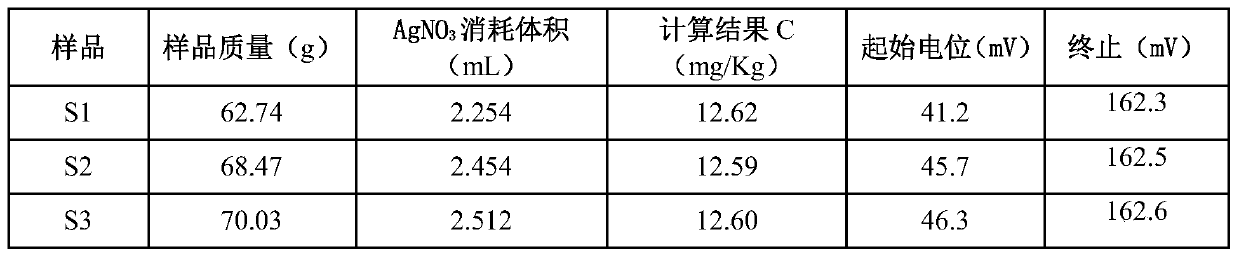
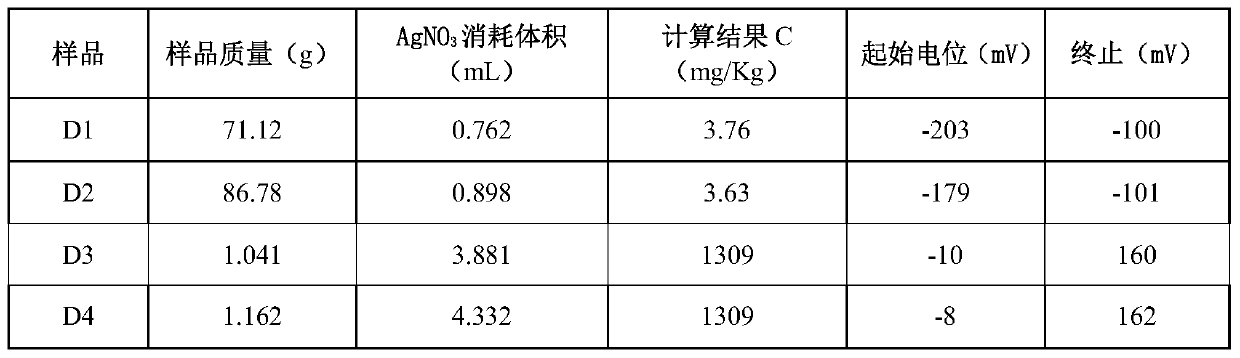
Chloride ion concentration determining method in nuclear power plant waste liquid treatment system and application thereof
InactiveCN110108836AReduce labor intensityHigh measurement accuracyChemical analysis using titrationLiquid wasteNuclear power
The invention relates to the technical field of waste liquid treatment in a mega-kilowatt nuclear power plant, in particular to a chloride ion concentration determining method in a nuclear power plantwaste liquid treatment system and application thereof. The method comprises the following steps of providing a silver nitrate standard solution and a sodium chloride solution with the known concentration; determining the titer of the silver nitrate standard solution on the sodium chloride solution; weighing the mass of a sample to be tested; adding a proper amount of nitric acid; titrating the sample to be tested by a potentiometric titrator and by using the nitric acid standard solution as a titrant so as to obtain a titration curve and the volume of the consumed silver nitrate standard solution for the titration end point potential; and calculating the concentration of chloride ions in the sample according to the concentration of the silver nitrate standard solution, the titer, the massof the sample to be tested and the volume of the consumed silver nitrate standard solution. The method can be used for determining the chloride ion concentration in complicated components of a nuclear power plant TEU evaporator; the defect of inaccurate titration end point judgment during the manual titration is overcome; the work intensity of test personnel is reduced; and safety, convenience and high speed are realized.
Owner:LINGDONG NUCLEAR POWER +5
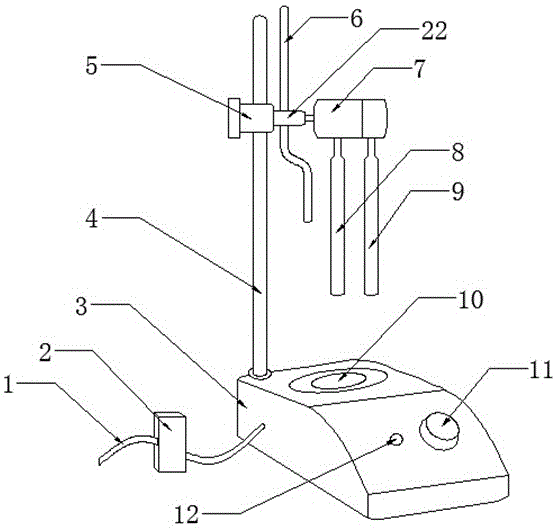
Automatic potentiometric titrator
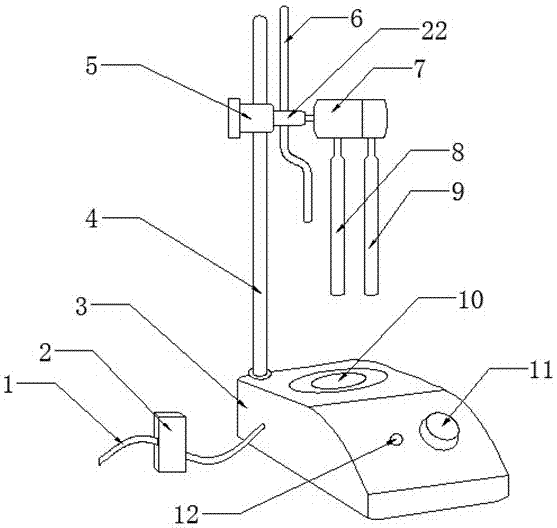
ActiveCN105806994ASave manpower and power resourcesIngenious designChemical analysis using titrationMaterial electrochemical variablesStopped workBurette
The invention provides an automatic potentiometric titrator which comprises a controller, a titrator body and a titration area, wherein an electric connecting wire is arranged on the outer surface of the titrator body; the controller is mounted on the electric connecting wire; a fixed rod is arranged at the rear end of the titrator body; a fixed head is mounted above the fixed rod; a solenoid valve is connected with the right end of the fixed head; a burette is mounted on the solenoid valve; an electrode fixing frame is connected with the right end of the solenoid valve; an indicator electrode and a reference electrode are welded under the electrode fixing frame; the burette, the indicator electrode and the reference electrode are arranged above the titration area; the titration area is arranged on the upper surface of the titrator body. The automatic potentiometric titrator has the beneficial effects that the titrator body can automatically start titration after a user puts a to-be-detected solution into a specific bottle and then places the bottle above the titration area, and the titrator body can automatically stop working after the completion of the titration, so that manpower and electric power resources are saved; the design is smart, the market competitiveness is promoted, and the purposes of simple structure and reasonable design are achieved.
Owner:上海昭晟机电(江苏)有限公司
Method for determining content of boron in boride
InactiveCN106770914AImprove accuracyEliminate judgmentChemical analysis using titrationBorideHydrogen
The invention belongs to the technical field of testing. A mathematic derivation method is adopted; a titration end point is accurately judged by utilizing pH (Potential of Hydrogen) value jump nearby the titration end point, probable errors are reduced and the testing accuracy is improved. A method for determining the content of boron in boride, provided by the invention, comprises the following steps of pre-treating a sample, titrating and processing data, wherein NaOH with the concentration of 0.01mol / L to 0.2mol / L as a titration solution; a pH value is collected by utilizing a pH meter and the collection interval is 10s to 50s; a titration curve takes a titration volume as a transverse coordinate and the pH value and a derivative thereof as a longitudinal coordinate; the maximum point of the derivative is used as the titration end point; the corresponding volume of the consumed NaOH solution is used as the volume of the titration end point; the contents of boron oxide, free boron and total boron are calculated respectively. The method provided by the invention can be used for eliminating judgment on the titration end point by artificial subjective factors and a testing result is accurate and reliable. The method is applicable to determination of the contents of the boron oxide, the free boron and the total boron in the boride.
Owner:SHANDONG NON METALLIC MATERIAL RES INST
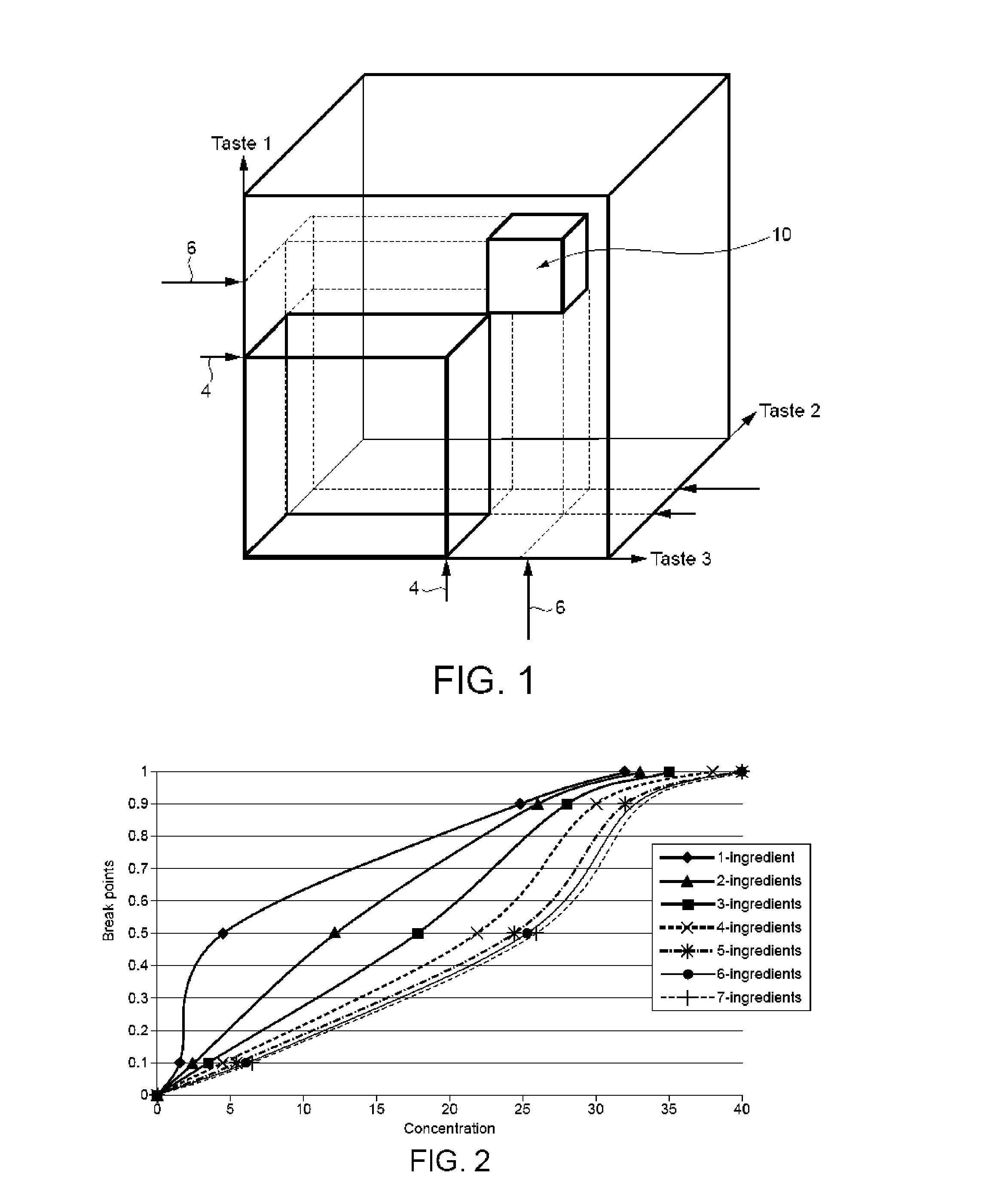
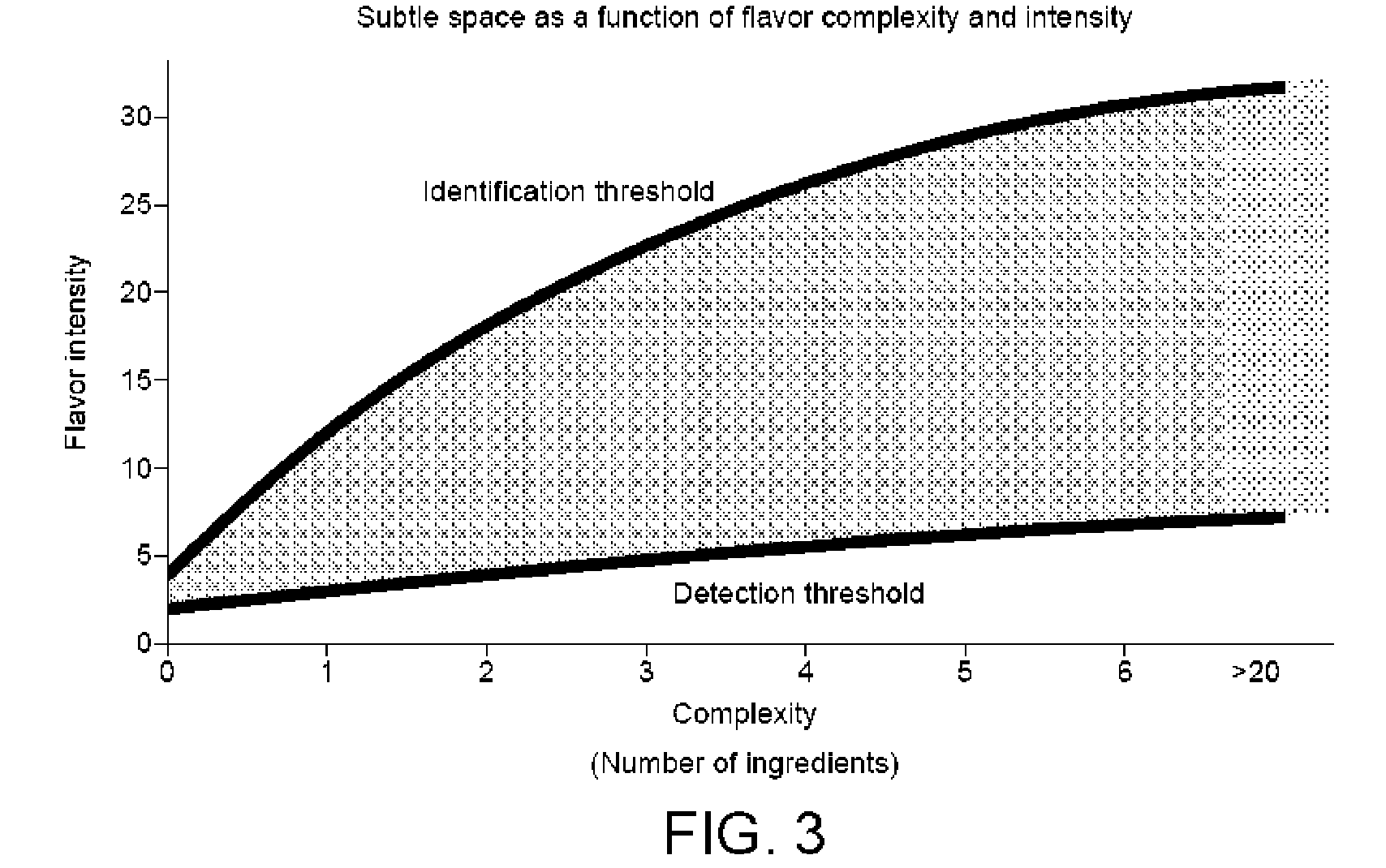
Method of creating flavour combinations and flavoured products
A method of developing a flavoured product comprises the steps of establishing a set of parameters in respect of a plurality of flavour components; selecting a platform for the product; selecting a group of flavour components based on objective requirements and the known established parameters; establishing for each of said flavour component relative to the selected platform at least two specific concentrations of the component in that platform relating to a human response in order to define a titration curve; measuring for a primary flavour component relative to that platform containing a predetermined concentration of each other flavour component the shift of said at least two specific concentrations; and utilising that shift information to restrict a number of measurements of the primary flavour component in the presence of additional flavour components in order to derive a range of concentrations for each component which lie between those specific concentrations. The method can implemented with the aid of a computer and databases to store flavour component data.
Owner:ZENDEGII
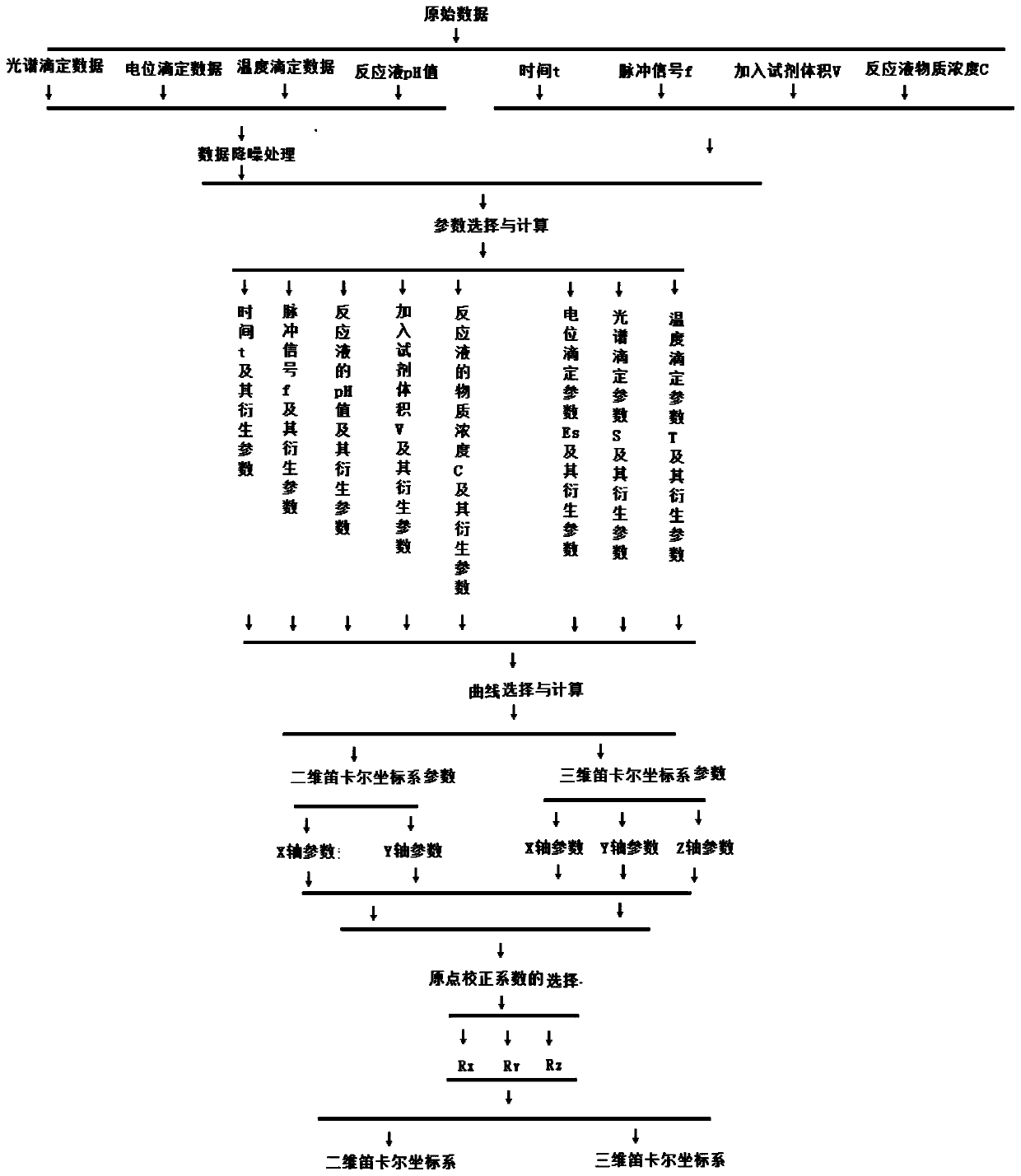
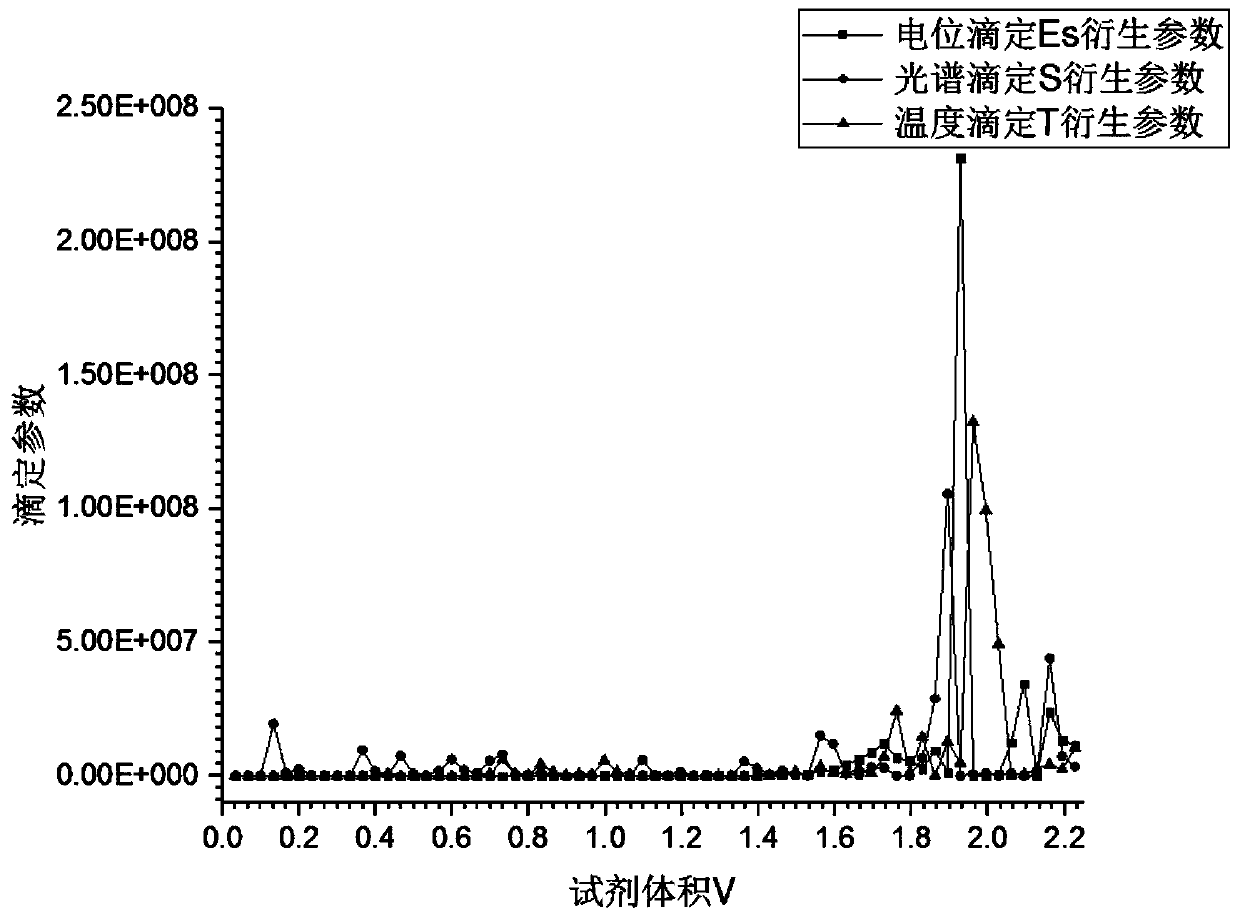
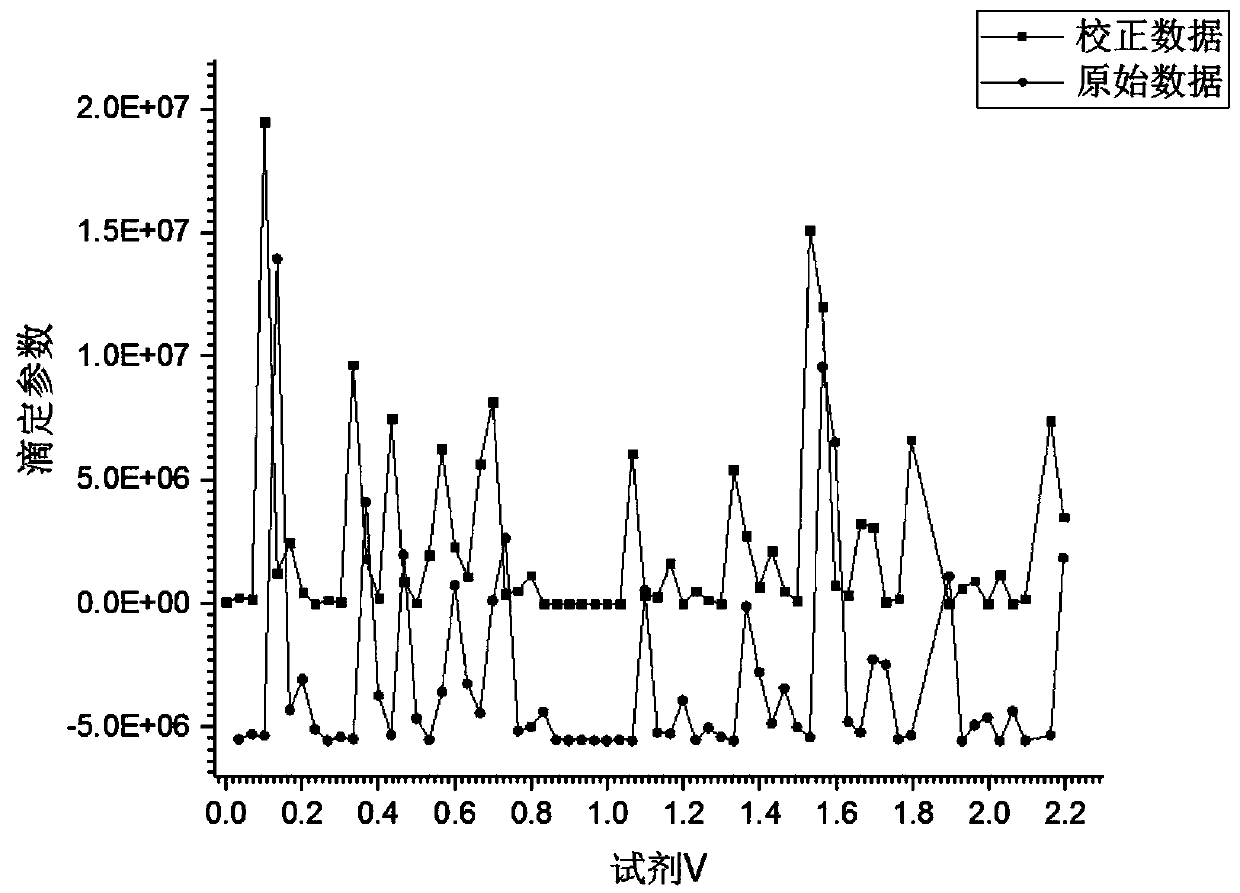
Data processing method for multi-dimensional titration analysis and application of data processing method
ActiveCN110632247AAchieve comparisonClear calculation methodChemical analysis using titrationTitration curveMulti dimensional
The invention discloses a data processing method for multi-dimensional titration analysis. According to the method, one or more measurement sequence parameters are set, so that the measurement benchmark of the titration analysis is expanded; one or more titration parameters are set, so that the synchronous measurement of multiple titration methods can be realized; original titration curves are constructed through the titration data; on the basis of the drift correction of the measurement sequence and titration data, the multiple original titration curves are unified at the same original pointof the same coordinate system, so that a corrected titration curve is obtained; the comparison of the multiple original titration curves in the same coordinate system is achieved; and therefore, the synchronous analysis of the measurement data of the different titration methods and the comparative analysis of the different measurement data are achieved. Furthermore, the invention also discloses application of the data processing method for the multi-dimensional titration analysis to the substance structure characterization and measurement of reaction solutions. With the data processing methodapplied, the comparative analysis of measurement data in different test processes can be realized.
Owner:王飞 +1
Method for testing density of polyacrylonitrile protofilament fibers
InactiveCN105954342AThe testing process is accurate and controllableParallel results are accurate and reliablePreparing sample for investigationMaterial electrochemical variablesJumping lineFiber bundle
The invention discloses a method for testing the density of polyacrylonitrile precursor fibers. Including: (1) preparation of iodine solution; (2) weighing the fiber bundle in the range of 0.09-0.41 grams; (3) putting the weighed fiber sample into a cylinder with a partition, and immersing the cylinder into (4) washing and centrifugal drying; (5) adding 80-100 milliliters of DMSO, stirring the sample until completely dissolved; (6) carrying out potentiometric titration on an automatic titrator with 0.1mol / L AgNO standard solution, The titration adopts linear titration, the titration speed is customized mode, the titration rhythm is 0.01ml / s, the sample titration jumps linearly as a curve, the titration curve is derived, and the titration volume corresponding to the maximum derivative value is used as the titration end volume; (7 ) to calculate the iodine absorption percentage. The test process of the present invention is accurate and controllable, requires standardization, and the obtained parallel results are accurate, reliable and highly comparable.
Owner:兰州蓝星纤维有限公司
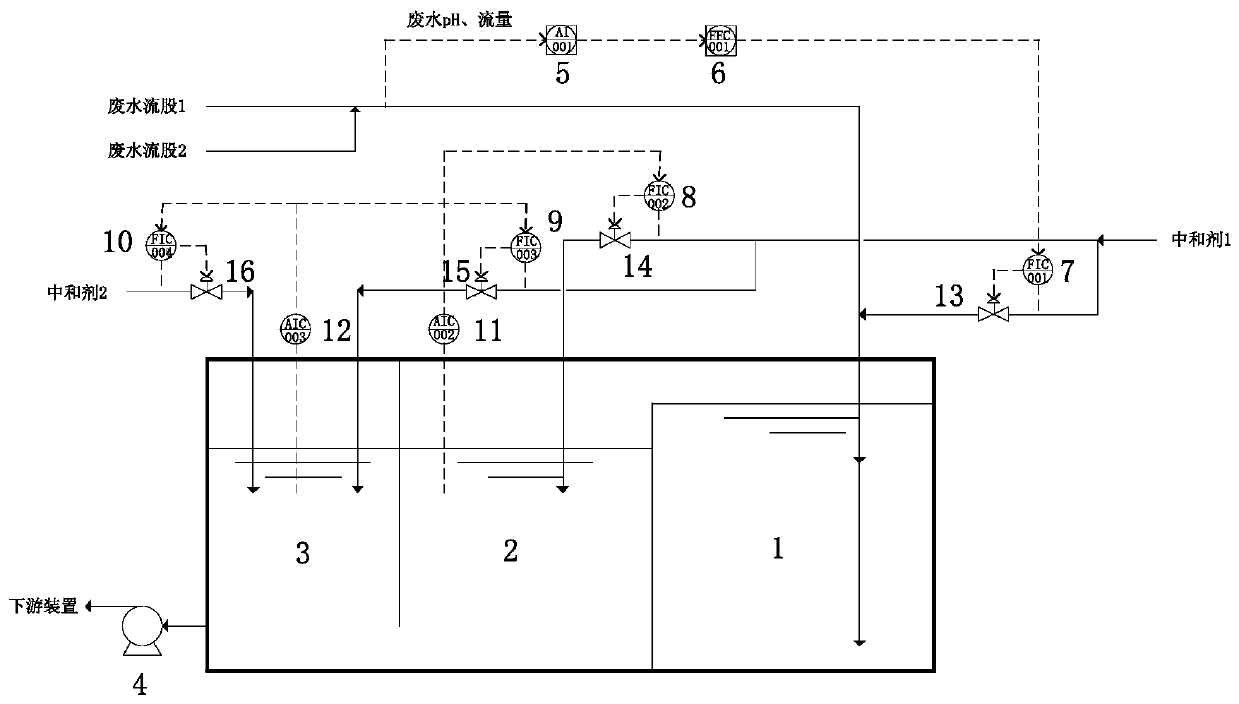
DCS based three-stage wastewater pH control method and system
ActiveCN110745932AReduce hysteresisAvoid hysteresisWater treatment parameter controlWater/sewage treatment by neutralisationPh controlWastewater
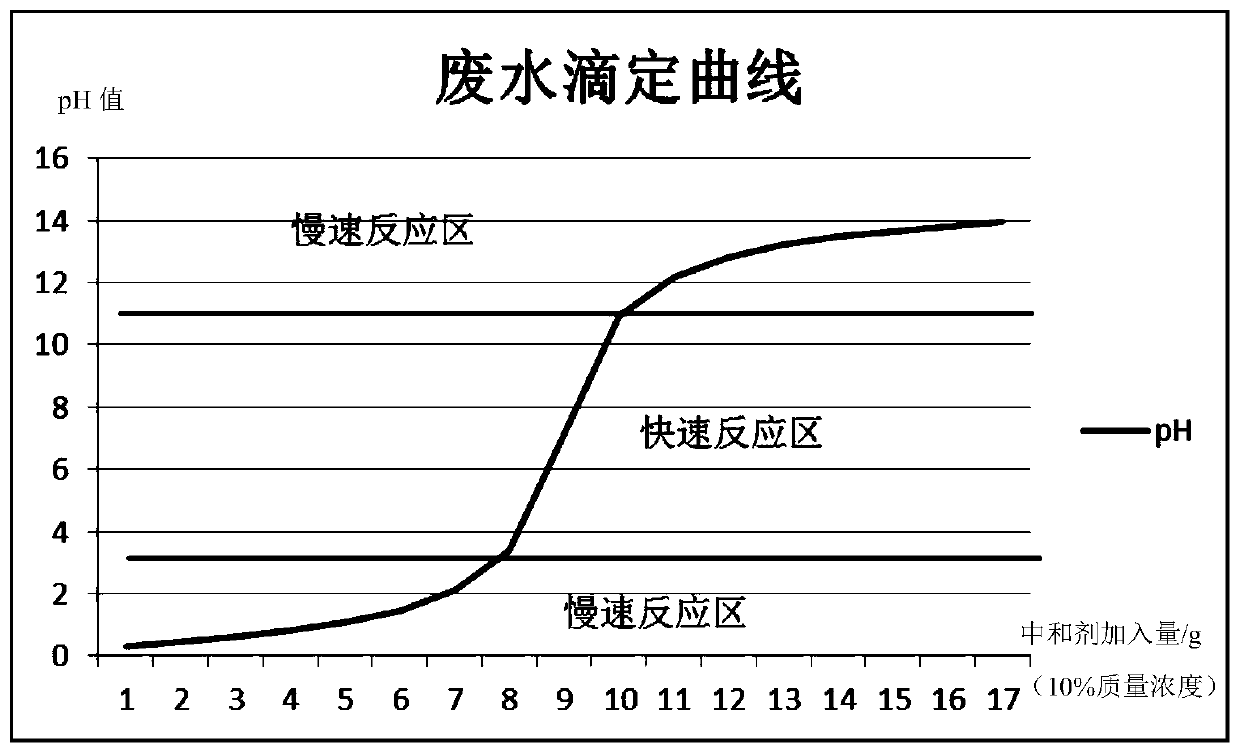
The invention discloses a DCS based three-stage wastewater pH control method and a system. The method comprises the following steps: (1) acquiring flow rate and a pH value of process effluent, a wastewater pH value of a fine adjustment tank and a wastewater pH value of a protection tank with tools, and carrying out communication with a DCS; (2) obtaining an acid / base amount entering a first-stagecoarse adjustment tank through a preset soft meter, multiplying the acid / base amount by a neutralizing proportional factor so as to obtain a set value of a neutralizer, and carrying out preliminary neutralization in the first-stage coarse adjustment tank; (3) continuing to add the neutralizer for adjustment according to a deviation between the wastewater pH value of the fine adjustment tank and the set value, and endowing a controller with different PID parameters in different intervals of a titration curve according to the wastewater pH value; and (4) continuing to add the neutralizer for adjustment according to a deviation between the wastewater pH value of the protection tank and the set value when the deviation exceeds a set interval. The method and the system have the aims that through multistage adjustment, the accuracy of wastewater pH adjustment is increased, the influence on processes and equipment of downstream devices caused by pH fluctuations is reduced, long-cycle stable operation of the devices is guaranteed, and the economic benefit is obvious.
Owner:WANHUA CHEM GRP CO LTD +1
Potentiometric titration instrument
InactiveCN1176372CAccurate judgmentImprove titration accuracyChemical analysis using titrationMaterial testing goodsAutomatic controlPetroleum product
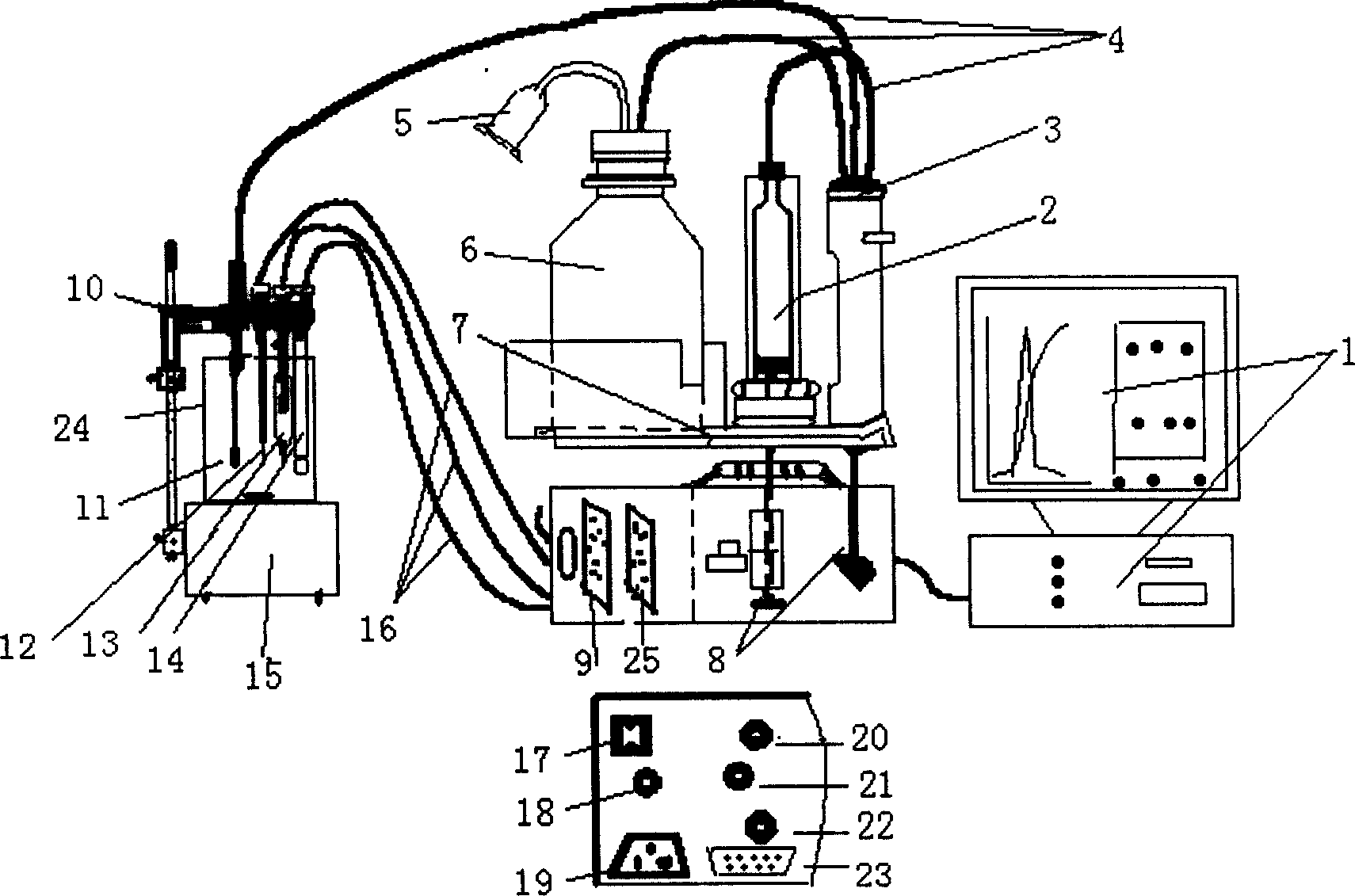
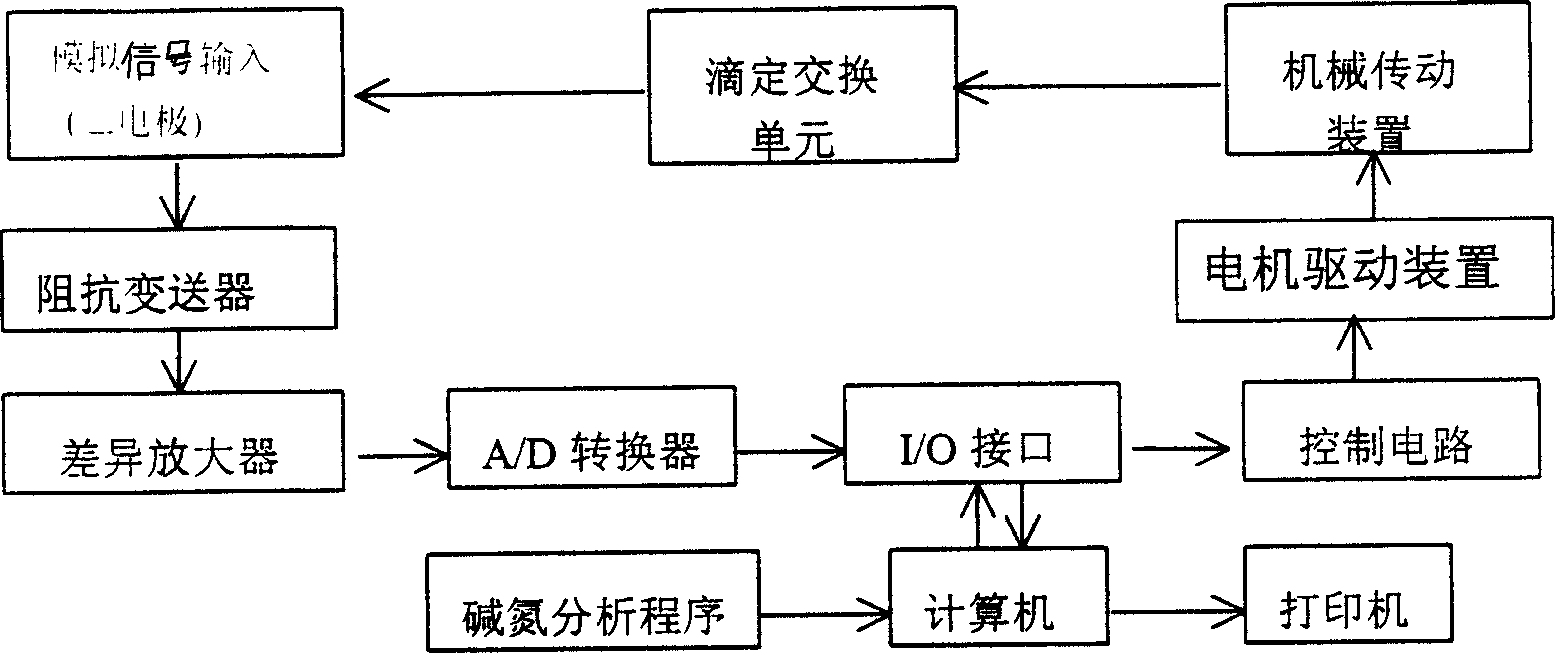
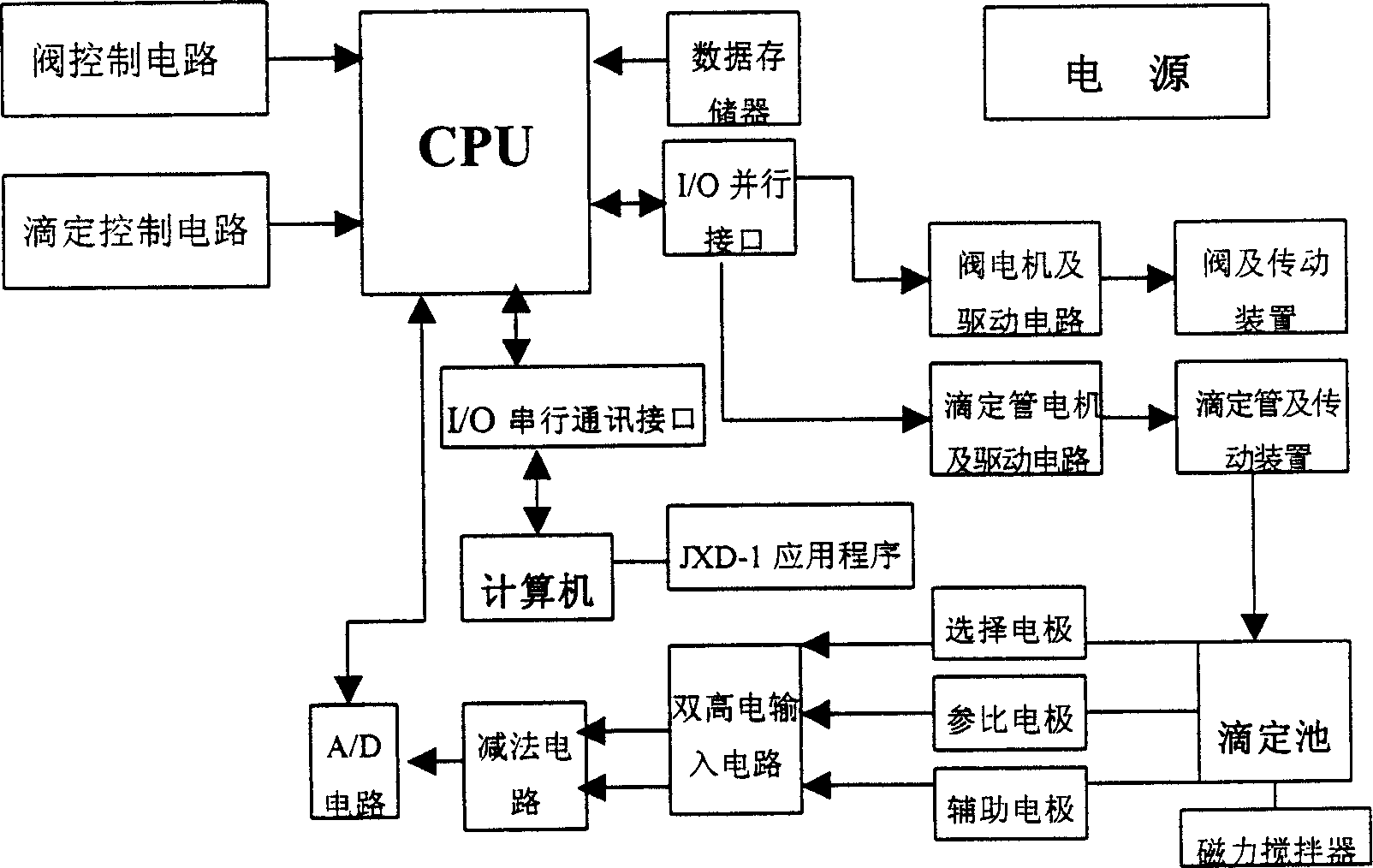
The potential titrator includes liquid sucking titration unit, data collecting and converting unit, driving unit, computer and application software. It has modular structure, in which the system unit and computer are connected serially for communication and the whole control process and data processing are performed via software automatically. The titrator can display real-time titration curve, terminate titration and display test data automatically, and is suitable for determining alkaline nitrogen content in petroleum and petroleum product and the potential titration of other specimens.
Owner:CHINA PETROLEUM & CHEM CORP +2
Quantum dot-encoded bead set for calibration and quantification of multiplexed assays, and methods for their use
InactiveUS20080131906A1Improved determinationMicrobiological testing/measurementNanoinformaticsAnalyteQuantum dot
Control beads are disclosed that allow for improved quantitation of analytes in multiplexed bead assays. The control beads have a range of concentrations of calibration moieties that provide for the preparation of a titration curve. The titration curve can be used to quantify the concentration of the analytes. The titration curve can be used to correlate the signal obtained from a bead with the concentration (or absolute number of molecules) of the analyte bound to the bead.
Owner:LIFE TECH CORP
Method and device for automatic determination of lime activity by titration
ActiveCN102798694BAutomate operationRealize instant controlChemical analysis using titrationBuretteWater baths
The invention relates to a method and a device for automatic determination of lime activity by titration, and the device comprises a titration rack, a burette, a flowmeter, a titration cup, a stirrer, a PH electrode, a titration control device, a titration table, and a water-bath heater. The burette is connected with a titrant bottle, and is provided with a titration switch. The titration control device is respectively connected with the PH electrode, the flowmeter and the titration switch circuit. The determination comprises the following steps: (1) accurately weighing 50.0 g of samples with a particle size of 1-5 mm, adding 4 mol / L hydrochloric acid into the titrant bottle; (2) setting the titration control device; (3) measuring 2000 ml water into the titration cup, stirring and heating to 40 DEG C; (4) adding the sample into the titration cup for digestion, starting the device for automatic titration; (5) displaying the consumption amount of the hydrochloric acid consumed by the titration. According to the invention, the PH value is used for titration control; the digestion reaction and the neutralization reaction are completed synchronously; precision of the hydrochloric acid amount is realized; the accuracy for lime activity determination is improved; and multifunctional analysis of the test data is realized through the titration control device.
Owner:SHIJIAZHUANG XINHUA IND FURNACE CO LTD
Rapid titration analysis method for N-methylglycine and salts thereof
InactiveCN103575777AFast and accurate titrationOvercome subjectivityMaterial electrochemical variablesStrong acidsPeak value
The invention discloses a rapid titration analysis method for N-methylglycine and salts thereof, relates to a rapid titration analysis method for free acids, carboxylic acids and amino acids in an aqueous solution, and belongs to the field of quantitative analysis. Volumes corresponding to first-order derivative peak values of a titration curve are determined as titration end points. The method comprises the following steps of adjusting a pH value of a solution to be less than 2 by using strong acids during titration, performing titration by using a standard alkali solution, generating a first peak value indicating that free strong acids are titrated when the pH value is 6 to 7, generating a second peak value indicating that the carboxylic acids are titrated when the pH value is 10.5 to 11.5, adding carbon disulfide to realize the DTF (dithio-formylation) reaction of a secondary amine group in the N-methylglycine, continuing performing titration by using the standard alkali solution to generate a third peak value until the pH value is 9.5 to 10.5, and calculating the free acid content, carboxylic acid content and amino acid content of the solution respectively. The method can be conveniently and quickly operated, and is low in analysis cost, and a reliable result can be obtained.
Owner:CHINA NAT OFFSHORE OIL CORP +2
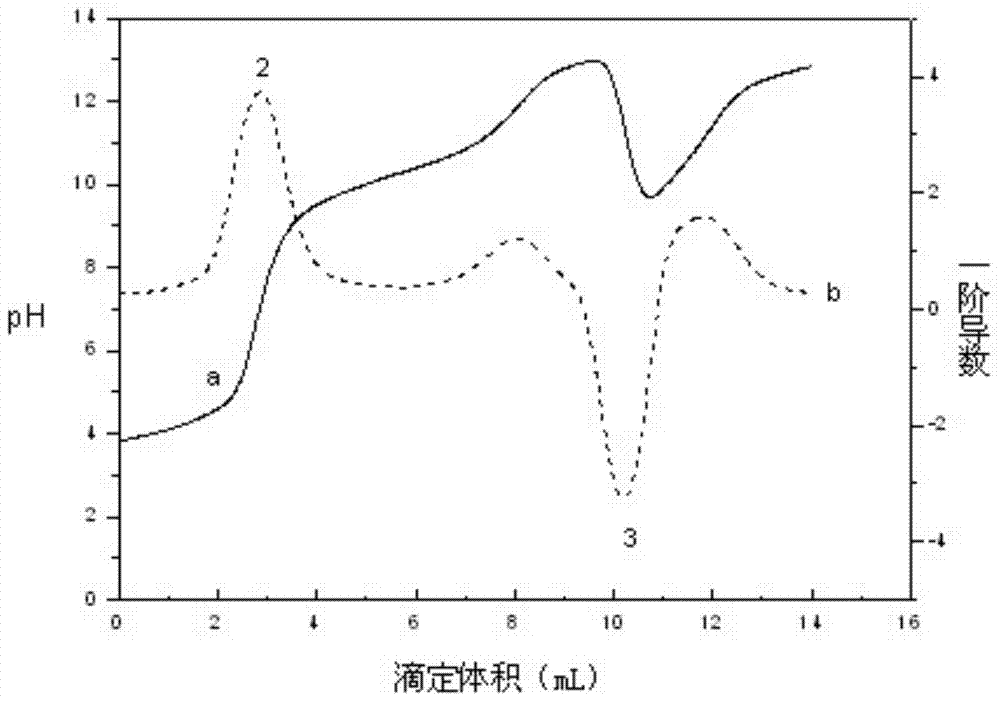
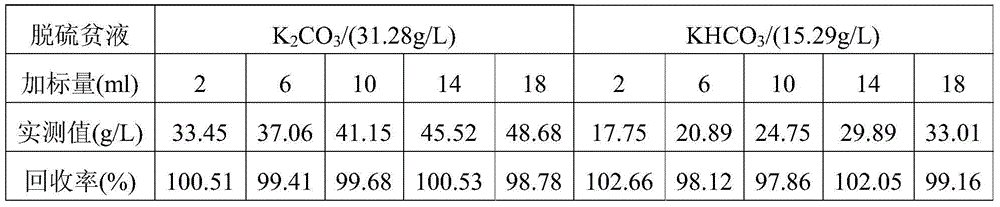
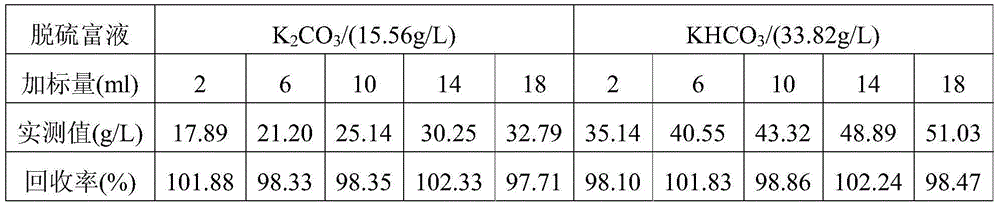
Method for detecting contents of potassium carbonate and potassium bicarbonate in desulfuration solution
InactiveCN104090015AImprove accuracyImprove stabilityMaterial electrochemical variablesChemical reactionPotentiometric titration
The invention provides a method for detecting the contents of potassium carbonate and potassium bicarbonate in a desulfuration solution. The method comprises the steps of preparing a sample solution of a desulfuration solution; by using an automatic potentiometric titrator, titrating the sample solution through a hydrochloric acid standard solution, and judging an equivalent point of a chemical reaction by using an inflection point of a potentiometric titration curve according to the jump of a potential of an electrode so as to figure out the contents of potassium carbonate and potassium bicarbonate in the sample solution by using an equation (1) and an equation (2), namely c(K2CO3)=c(HCl)*V1*138 / V, and c(KHCO3)=c(HCl)*(V2-2V1)*100 / V. According to The method for detecting the contents of potassium carbonate and potassium bicarbonate in the desulfuration solution, provided by the invention, the operation is simple and convenient, an indicator is not needed, and the accuracy and the stability of a detection result are high because the judgment of a titration end point cannot be disturbed by the color of the desulfuration solution.
Owner:PANGANG GRP XICHANG STEEL & VANADIUM CO LTD
Method for detecting 1-hydroxy ethylene-1, 1-diphosphonic acid
InactiveCN1670510AReduce dosageLow priceChemical analysis using titrationMaterial analysis by observing effect on chemical indicatorTitration curveTest light
This invention relates to hydroxyl-ethidene diphosphoric acid test method, which comprises the following steps: adding buffer, shield and indicating agents into the HEDP solution and dripping solution to test the light absorption degree and make dripping curve and figure out dripping agent volume V and make standard working curve; adding buffer, shield and indicative agents in the sample to test light absorption degree to make dripping curve and to figure out volume Vx, and to figure out the HEDP concentration by the standard working curve; the concentration degree multiplies the diluting times to get the effective content of HEDP.
Owner:XIAMEN UNIV +1
An automatic potentiometric titrator
ActiveCN105806994BSave manpower and power resourcesIngenious designChemical analysis using titrationMaterial electrochemical variablesStopped workBurette
The invention provides an automatic potentiometric titrator which comprises a controller, a titrator body and a titration area, wherein an electric connecting wire is arranged on the outer surface of the titrator body; the controller is mounted on the electric connecting wire; a fixed rod is arranged at the rear end of the titrator body; a fixed head is mounted above the fixed rod; a solenoid valve is connected with the right end of the fixed head; a burette is mounted on the solenoid valve; an electrode fixing frame is connected with the right end of the solenoid valve; an indicator electrode and a reference electrode are welded under the electrode fixing frame; the burette, the indicator electrode and the reference electrode are arranged above the titration area; the titration area is arranged on the upper surface of the titrator body. The automatic potentiometric titrator has the beneficial effects that the titrator body can automatically start titration after a user puts a to-be-detected solution into a specific bottle and then places the bottle above the titration area, and the titrator body can automatically stop working after the completion of the titration, so that manpower and electric power resources are saved; the design is smart, the market competitiveness is promoted, and the purposes of simple structure and reasonable design are achieved.
Owner:上海昭晟机电(江苏)有限公司
Method for measuring alkalinity of surfactant based on potentiometric titration method
PendingCN113702473ASolve the problem of inaccurate judgment of titration end point using photometric electrodeSolve the problem of inaccurate judgmentMaterial analysis by electric/magnetic meansAlkalinityComposite electrode
The invention discloses a method for determining alkalinity of a surfactant based on potentiometric titration, and relates to the technical field of surfactant performance determination. The method comprises the following steps: weighing 3-5g of a sample, adding the sample into 100mL of water, placing the water on a high-precision photo-thermal potential analyzer, adding a magnetic rotor, and uniformly stirring; selecting dynamic titration as a titration method, wherein the maximum void volume is 0.4 mL, and the minimum void volume is 0.02 mL; and using a pH composite electrode, after the electrode and a feed tube are immersed into the liquid level, using the prepared 0.1 mol / L hydrochloric acid titration solution for titration to the end point, and the titration volume is read. The pH composite electrode is adopted, the titration end point is judged through potential jump, an indicator does not need to be added into a titration system, the titration end point is judged by detecting a jump point of a titration curve through the potential electrode, and judgment of the titration end point is more accurate; and the problem that the titration end point of a dark or lightproof sample is inaccurately judged by using a photometric electrode is solved.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Method for determining hydrolysis degree of partially hydrolyzed polyacrylamide for oil displacement
PendingCN112881588ACalculation of degree of hydrolysisProduct quality is easy to controlChemical analysis using titrationPartial hydrolysisPolymer solution
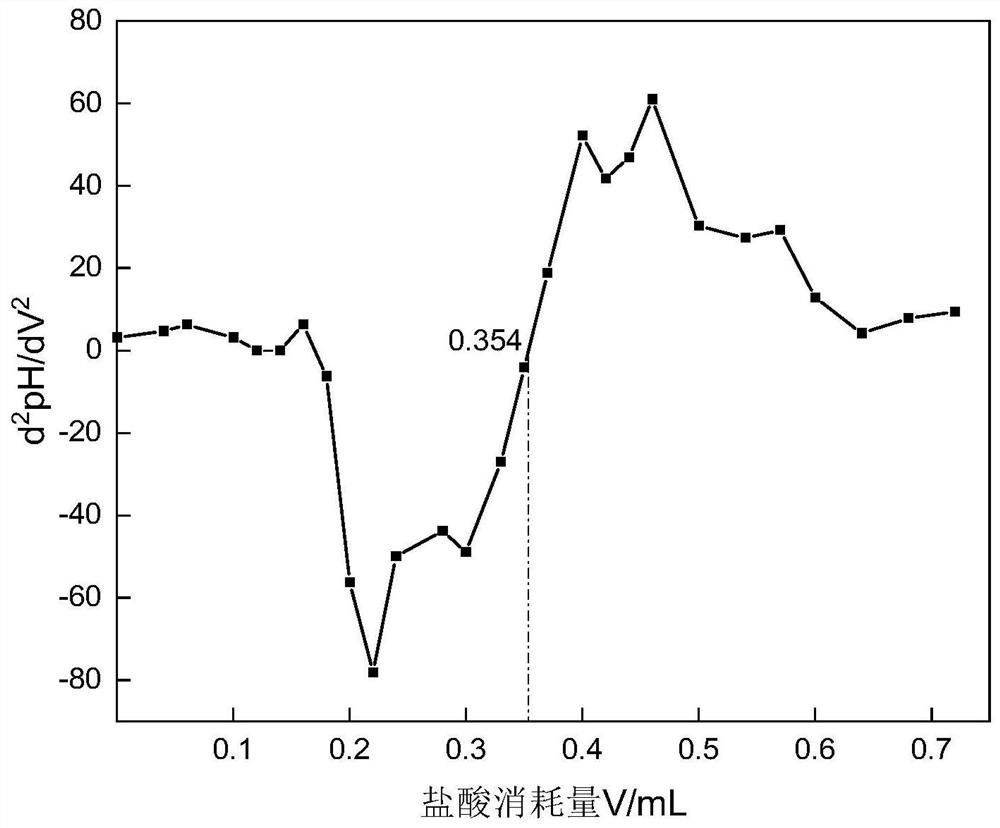
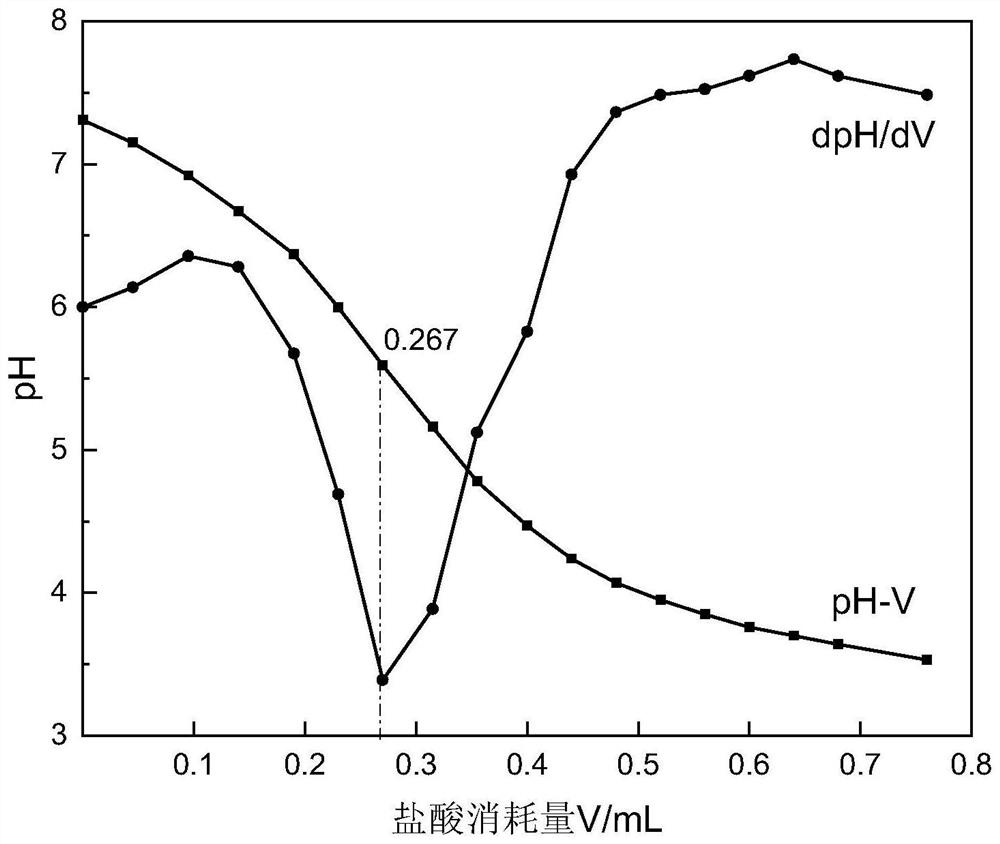
The invention relates to a method for determining the hydrolysis degree of partially hydrolyzed polyacrylamide for oil displacement. The method mainly solves the problem that the color of a titration end point is difficult to recognize due to the fact that an indicator in a polymer solution does not have an obvious color mutation point in a titration process in an existing determination method and man-made subjective judgment errors are large. The method is characterized by comprising the steps of (1) measuring the solid content of a polymer sample, and preparing a solution to be measured according to the solid content; (2) dropwise adding a hydrochloric acid standard solution into the prepared solution to be detected, measuring the pH value of the solution, and stopping titration when the pH value reaches 3.5; (3) drawing a pH-V titration curve; and (4) solving a first derivative and a second derivative of the pH-V titration curve, taking the hydrochloric acid consumption corresponding to the maximum point of the obtained first derivative curve and the second derivative d2pH / dV2 being 0 as a titration end point, and calculating to obtain the hydrolysis degree of the sample. According to the method, personal errors caused by unobvious color change of the solution can be basically eliminated, and the detection accuracy and precision are improved.
Owner:DAQING OILFIELD CO LTD +1
Petroleum product base number detection method, detection device and application thereof
InactiveCN111707779ABase number calculationImprove detection accuracyChemical analysis using titrationMaterial electrochemical variablesPetroleum productPhysical chemistry
The invention discloses a petroleum product base number detection method, a detection device and application thereof. The invention relates to the technical field of lubricating oil detection and evaluation. Specifically, in the detection method, a titration end point of a petroleum product is set at a pH value of -4.5 to -5.5 and at a potential value of 620-680mv, and under the condition that extra test steps and extra reagents do not need to be added, according to the volume of a titrant used when the petroleum product reaches a fixed titration end point, the base value of the petroleum product can be accurately calculated even for a petroleum product of which the end point of an inflection-free titration curve cannot be accurately judged. The method has the advantages of higher detection accuracy and higher automation degree.
Owner:GUANGZHOU MECHANICAL ENG RES INST
Titrimetric analysis method
InactiveCN105651932AImprove general performanceEasy to operateChemical analysis using titrationChemical reactionWorking temperature
The invention relates to a titrimetric analysis method and belongs to the field of inorganic chemical analysis. The titrimetric analysis method comprises the following steps: preparing a standard titrating solution and a standard titrated solution; confirming the volume of a titrated solution sample, distribution rate of a titrating solution and working temperature; using a non-contact high-frequency electronic sensor for monitoring the change in conductivity of a reaction liquid in a titrimetric reaction in real time; taking an inflection point on a titration curve as a titration end point; establishing a relation function between the time required by reaching the titration end point and the concentration of the titrated solution; and measuring the titration time for reaching the titration end point of the to-be-measured sample under same parameters and substituting the titration time into the working curve, thereby acquiring the concentration data. The titrimetric analysis method is strong in universality and simple in operation; the electrode does not need to be exchanged and pretreated; the auxiliary facilities for stirring, controlling temperature, and the like, are not required in the titrating process; the method is applicable to four inorganic chemical reactions related to acid-base, sediment, complexing and redox; the sensitivity is high; and the method can be used for detecting the microliter-scale sample.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com