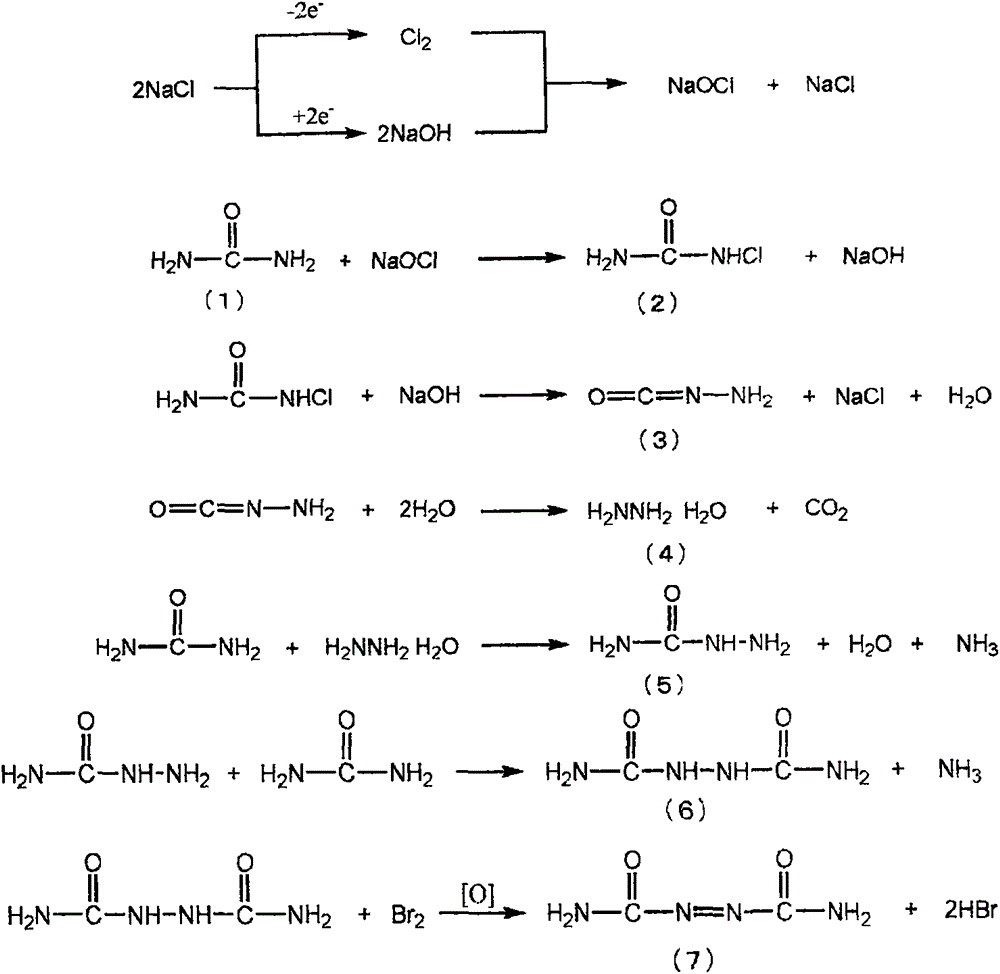
New production method for azodicarbonamide
A technology of azodicarbonamide and manufacturing method, which is applied in electrolysis process, organic chemistry, electrolysis components, etc., can solve the problems of economical benefit and waste of price electricity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0084] Hereinafter, the present invention will be described in more detail using examples, but the present invention is not limited to these examples.
[0085] The azodicarbonamide obtained in each of the following examples passes through infrared absorption spectrum (IR) and proton nuclear magnetic resonance spectrum ( 1 H-NMR) for identification.
[0086] IR(KBr): 3337, 3187cm -1 (N-H telescopic), 1743, 1731cm -1 (C=O expansion and contraction), 1637cm -1 (N-H variable angle), 1369, 1331cm -1 (C-N telescopic).
[0087] 1 H-NMR(D6-DMSO): δ8.02(s, 2H, NH), 7.97(s, 2H, NH).
[0088] Quantification of unreacted urea in each of the following examples was performed by high performance liquid chromatography.
[0089] The analysis conditions of the high performance liquid chromatography are as follows.
[0090] column:
[0091] Mobile phase: 10mM acetic acid in water
[0092] Flow rate: 1ml / min
[0093] Detection wavelength: 205nm
[0094] The conversion rate in the fo...
reference example 1
[0099] In a reaction vessel equipped with a stirrer and a thermometer, 75 g (1.25 mol) of urea was dissolved in 150 ml of water, and 321 ml (1.25 mol) of 29% sodium hypochlorite aqueous solution was added dropwise at 10° C. with stirring over 1 hour. Take a sample, add potassium iodide and acetic acid (1:1), and quickly carry out 0.1N-sodium thiosulfate titration. As a result of titration analysis, it was confirmed that the yield of monochlorourea was 98.4%. When ultraviolet analysis was performed, absorption at 244 nm peculiar to monochlorourea was confirmed.
reference example 2
[0101] Based on the addition of 10.7% NaOCl aqueous solution (as Cl 2 The preparation of bromourea solution (NaOCl: HCl: urea: the molar ratio of NaBr 1: 2.2: 37.7: 0.8) of 38% aqueous solution (weight %) of NaBr
[0102] In a 250ml round bottom flask equipped with a stirring bar, dropping funnel and thermometer, 46.04g of urea (767mmol) was dissolved in 30.6g of H 2 O, followed by the addition of 5.4 g of 32% HCl (47.2 mmol) (with heat generation upon cooling). A solution of urea hydrochloride was obtained. 13.5 g of 10.7% NaOCl aqueous solution (with Cl 2 % by weight, 20.3 mmol) was added to the addition funnel, and 4.84 g of 38% NaBr solution was added to another addition funnel. The NaOCl solution was added to the urea hydrochloride solution and 38% NaBr aqueous solution was added to this solution 1 min later. When ultraviolet analysis is carried out, bromureon-specific absorption at 275 nm is shown. Take a sample, add potassium iodide and acetic acid (1:1), and quick...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com