Polycarboxylic acid and polycarboxylic acid composition containing same, epoxy resin composition, thermosetting resin composition, and cured material of same, and optical semiconductor device
A technology of resin composition and polycarboxylic acid, which is applied in semiconductor devices, semiconductor/solid-state device parts, organic chemistry, etc., can solve problems such as inability to be suitable for mold forming, deviation of cured product properties, high melting point, etc., and achieve full vitrification Transition temperature, excellent resin reactivity, and excellent cured physical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0324] Embodiment 1: the manufacture of polyhydric carboxylic acid (A-1)
[0325] 26.1 g of tris(2-hydroxyethyl) isocyanurate, RIKACID MH-T (4-methylhexahydro Phthalic acid) 52.1 g, toluene 70 g, a serpentine double-helix condenser (Dimroth condenser), a stirring device, and a thermometer were installed, and the flask was immersed in an oil bath. The oil bath was heated, and the internal temperature was kept at 115° C., and the reaction was carried out for 7 hours as it was.
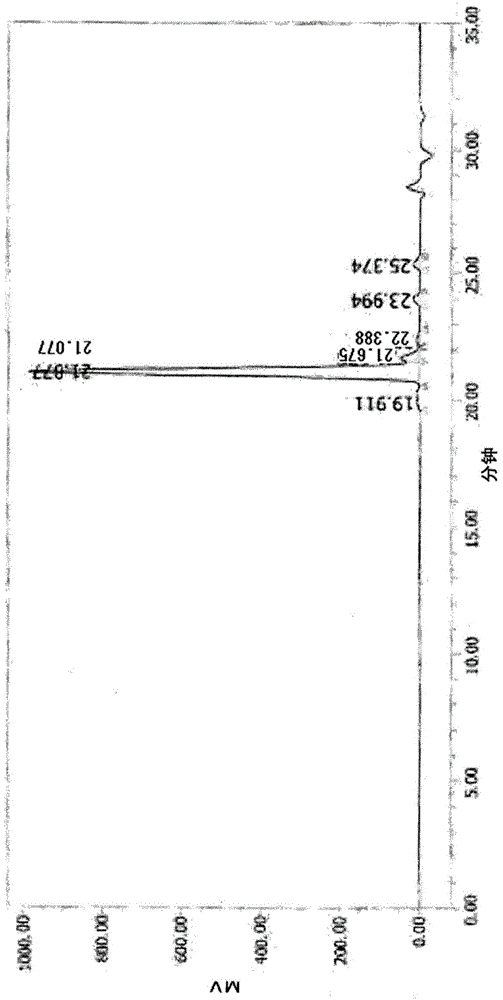
[0326] The obtained reaction liquid was concentrated under reduced pressure at 100° C., and toluene was distilled off to obtain 71.5 g of a polyvalent carboxylic acid (A-1) having the following formula (23) as a main component. The GPC purity (GPC area %) of the obtained compound was 92%, the acid value was 203.4 mgKOH / g, and the external appearance was a white solid. In addition, the melting point (peak apex value) using DSC was 57.0°C, and the thermogravimetric reduction was -3.4%. The GPC chart of ...
Embodiment 2
[0328] Embodiment 2: Manufacture of polyhydric carboxylic acid composition (C-1)
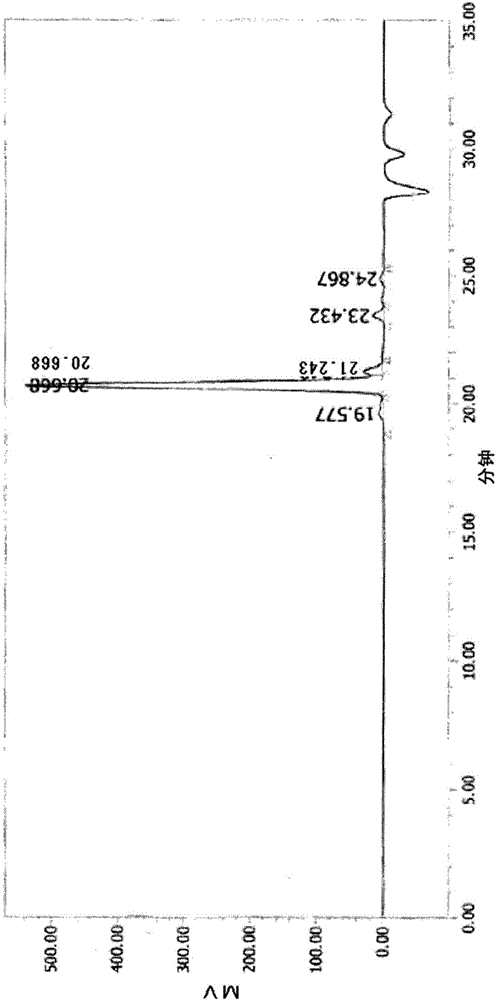
[0329]26.1 g of tris(2-hydroxyethyl) isocyanurate, RIKACID MH-T (4-methylhexahydro Phthalic anhydride) 123.4g, a serpentine double-helix condenser, a stirring device, a thermometer are set, and the flask is soaked in an oil bath. The oil bath was heated, and the internal temperature was kept at 78° C., and the reaction was carried out for 4 hours as it was. It was confirmed by GPC that the peak of tris(2-hydroxyethyl) isocyanurate was 1 area % or less, and 147 g of a polyvalent carboxylic acid composition (C-1) belonging to a mixture of a polyvalent carboxylic acid and a carboxylic anhydride compound was obtained. The resulting mixture is a colorless and transparent liquid, and the purity measured by GPC is 59.5 area % of the polycarboxylic acid (A-1) represented by the aforementioned formula (23), 4-methyl Hexahydrophthalic acid accounted for 1.3 area%, and 4-methylhexahydrophthalic anhydride...
Embodiment 3
[0331] Embodiment 3: the manufacture of polycarboxylic acid composition (C-2)
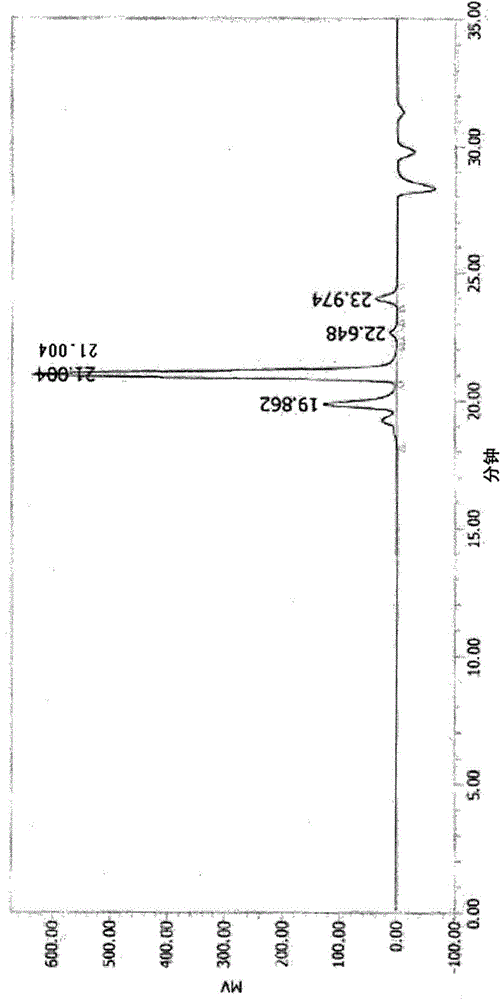
[0332] In a container made of polypropylene, 20 g of the polyvalent carboxylic acid composition (C-1) obtained in Example 2 and 6.67 g of RIKACIDMH-T (4-methylhexahydrophthalic acid manufactured by Shikoku Chemical Industry Co., Ltd.) were placed. , and mixed with a spatula to obtain 26.6 g of a polycarboxylic acid composition (C-2). The obtained mixture is a colorless and transparent liquid, and the purity measured by GPC is 46.2 area % of the polycarboxylic acid ((A-1) represented by the aforementioned formula (23), 4-methylhexahydrophthalic acid ( The aforementioned formula (24)) was 3.9 area %, and 4-methylhexahydrophthalic anhydride was 49.9 area %. In addition, the functional group equivalent was 187 g / eq, the viscosity was 24678 mPa·s, and the thermogravimetric reduction was -31.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com