Heterocyclicacyl iminethiazole compound as well as preparation method and application thereof
A kind of technology of heterocyclic acyl imino thiazole and compound, which is applied in the field of agricultural pesticides and can solve the problems of undisclosed heterocyclic acyl imino thiazole compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
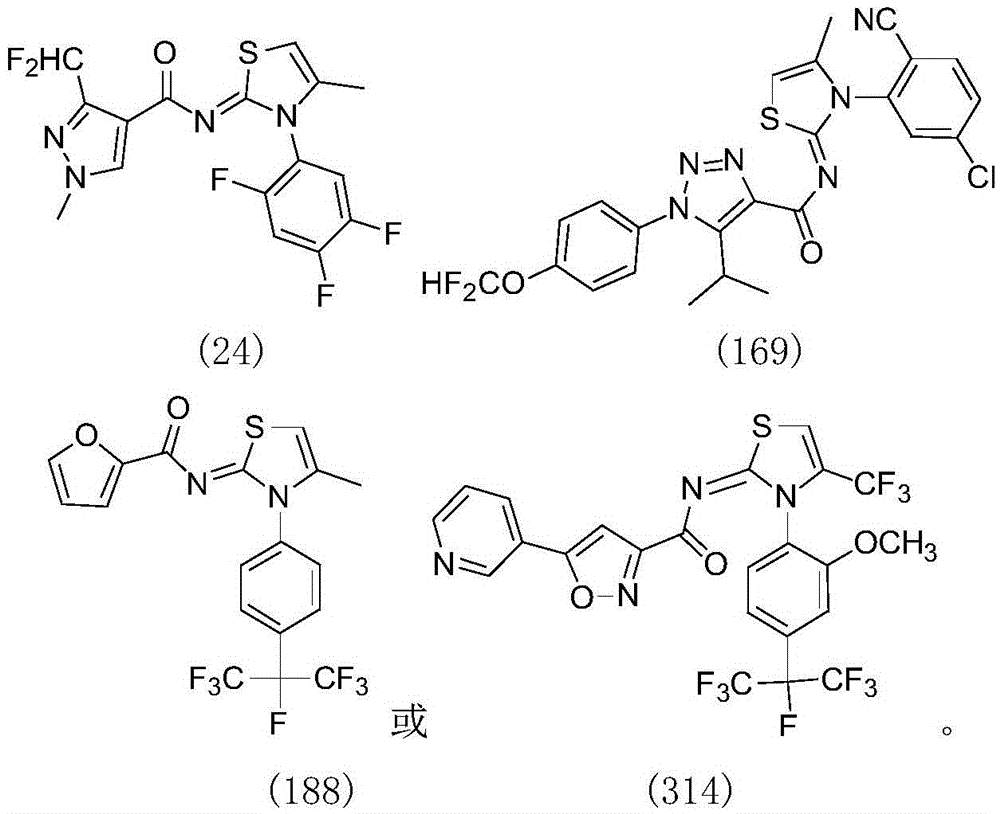
[0099] 3-Difluoromethyl-1-methyl-1H-pyrazole-4-carboxy-[4-methyl-3-(2,4,5-trifluorophenyl)-3H-thiazole-2-ylidene Preparation of ]-amide (Compound No. 24)
[0100] Step 1: Synthesis of 3-difluoromethyl-1-methyl-1H-pyrazole-4-carbonyl chloride
[0101]
[0102] Add 53.00g of 3-difluoromethyl-1-methyl-1H-pyrazole-4-carboxylic acid and 72mL of thionyl chloride to a single-necked flask, raise the temperature until the system refluxes, cool after reflux for 2 hours, and evaporate at normal pressure Unreacted thionyl chloride. The temperature of the system is lowered to below 60°C, an anhydrous calcium chloride drying tower is connected externally, and the low-boiling impurities are evaporated under reduced pressure by a water pump to obtain a red viscous liquid, which solidifies into white crystals overnight. Yield 78.25%.
[0103] The NMR data of white crystals are: 1 H NMR (400MHz, CDCl 3 ),δ:3.95(s,3H,N-CH 3 ),6.97(t,J H-F =54.20Hz,1H,-CHF 2 ), 8.06 (s, 1H, pyrazolyl-H...
Embodiment 2
[0114] 2-[2-(3-Chloro-pyridine)]-5-fluoromethoxy-2H-pyrazole-3-carboxy-[4-methyl-3-(2-methyl-4-heptafluoroiso Preparation of propyl-phenyl)-3H-thiazol-2-ylidene]-amide (compound 70)
[0115]
[0116] With reference to the reaction steps in Example 1, 1-{2- [2-(3-Chloro-pyridine)]-5-fluoromethoxy-2H-pyrazole-3-carbonyl}-3-(2-methyl-4-heptafluoroisopropyl-phenyl)-sulfur Urea, then reacted with bromoacetone to obtain the target product 2-[2-(3-chloro-pyridine)]-5-fluoromethoxy-2H-pyrazole-3-carboxy-[4-methyl-3-( 2-Methyl-4-heptafluoroisopropyl-phenyl)-3H-thiazol-2-ylidene]-amide, yield: 65.2%.
Embodiment 3
[0118] 1-(4-Difluoromethoxy-phenyl)-5-isopropyl-1H-[1,2,3]triazole-4-carboxy-[3-(5-chloro-2-cyano- Preparation of phenyl)-4-methyl-3H-thiazol-2-ylidene]-amide (compound 169)
[0119]
[0120] Referring to the reaction steps in Example 1, using 1-(4-difluoromethoxy-phenyl)-5-isopropyl-1H-[1,2,3]triazole-4-carboxylic acid as raw material 1-(5-chloro-2-cyano-phenyl)-3-[1-(4-difluoromethoxy-phenyl)-5-isopropyl-1H-[1,2,3] Triazole-4-carbonyl]-thiourea, then reacted with bromoacetone to obtain the target product 1-(4-difluoromethoxy-phenyl)-5-isopropyl-1H-[1,2,3]tri Azole-4-carboxy-[3-(5-chloro-2-cyano-phenyl)-4-methyl-3H-thiazol-2-ylidene]-amide, yield: 66.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com