A stable serum iron detection kit
A technology for detecting kits and serum iron, which can be used in the measurement of color/spectral characteristics, material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, etc. It can solve the problems of high iron toxicity, etc. To achieve the effect of enhanced stability, cheap price and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A conventional kit for detecting serum iron, which includes reagent R1 and reagent R2.
[0030] Wherein the reagent R1 consists of:
[0031] pH 4.2 Tris buffer 60mmol / L
[0032] Ascorbic acid 100mmol / L
[0033] Sodium azide 10mmol / L
[0034] Surfactant TritonX-100 1%
[0035] Reagent R2 consists of:
[0036] pH 4.2 Tris buffer 60mmol / L
[0037] Ferrozine 50mmol / L
[0038] Sodium azide 10mmol / L
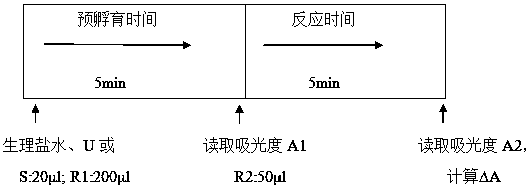
[0039] The reagent R1 and reagent R2 described in this embodiment need to be prepared by adding the buffer substance Tris first, and then adding other substances after adjusting the pH to an appropriate pH value with hydrochloric acid. The test kit described in this embodiment, when in use, is to use Mindray 800 full-automatic biochemical analyzer with double reagent function, and utilize the endpoint method to measure, the operation is as follows:
[0040] Add 20 μl of physiological saline, sample or calibrator, then add 200 μl of R1 reagent for pre-incubation for 5 min...
Embodiment 2
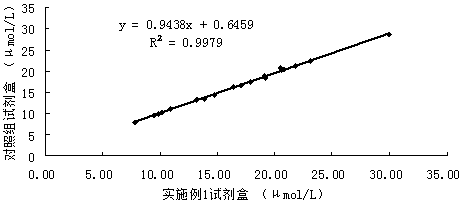
[0045] Accuracy verification test: the serum iron detection reagent of Example 1 was used as the experimental group, and a serum iron detection kit with good accuracy and stability recognized on the market was used as the control group for detection, and 20 clinical serum samples were tested The test was carried out, and the test results are shown in Table 1. Correlation curves for the two reagents were obtained (eg figure 1 Shown), the results show that the correlation coefficient of the two kits is 0.9989, indicating that the correlation between the two is relatively good. It proves that the added and changed components of the kit of the present invention will not affect its accuracy, and the kit still maintains a good accuracy.
[0046] Table 1 Example 1 reagent and market-approved serum iron detection kit comparative detection results
[0047]
Embodiment 3
[0049]Linear correlation verification test: Prepare a high-value sample with an iron content of 180 μmol / L, serially dilute it with normal saline, and prepare 6 samples with different concentrations, the concentrations are 180 μmol / L, 144 μmol / L, 108 μmol / L, 72 μmol / L L, 36 μmol / L, 0 μmol / L. The reagents of Example 1 and the control group were used for detection respectively. The samples of each concentration were measured three times, and the average values were taken respectively. The detection results are shown in Table 2.
[0050] Table 2 The detection results of the reagent linear correlation verification experiment in Example 1
[0051]
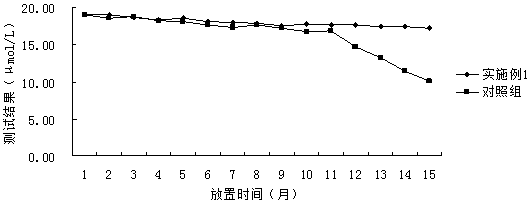
[0052] As shown in the above table, the correlation coefficients of embodiment 1 and the detection results of the control group reagents are all greater than 0.990, and the correlation coefficients of the detection results of the reagents of embodiment 1 are slightly greater than the correlation coefficients of the detection result...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dominant wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com