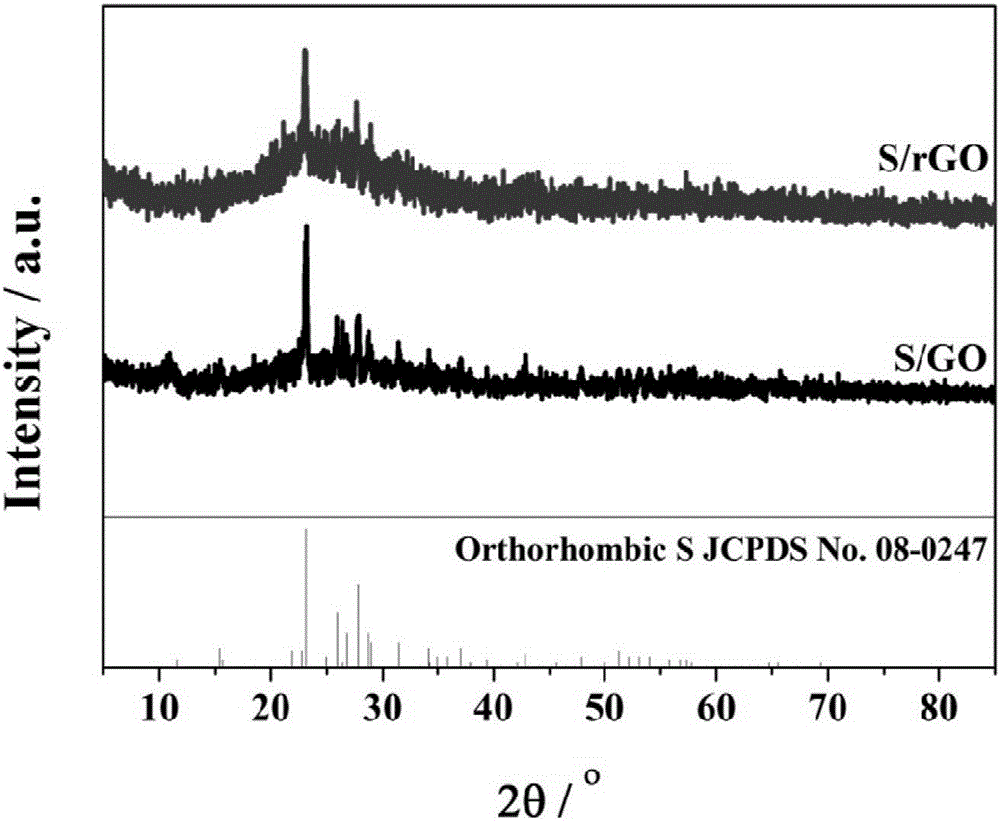
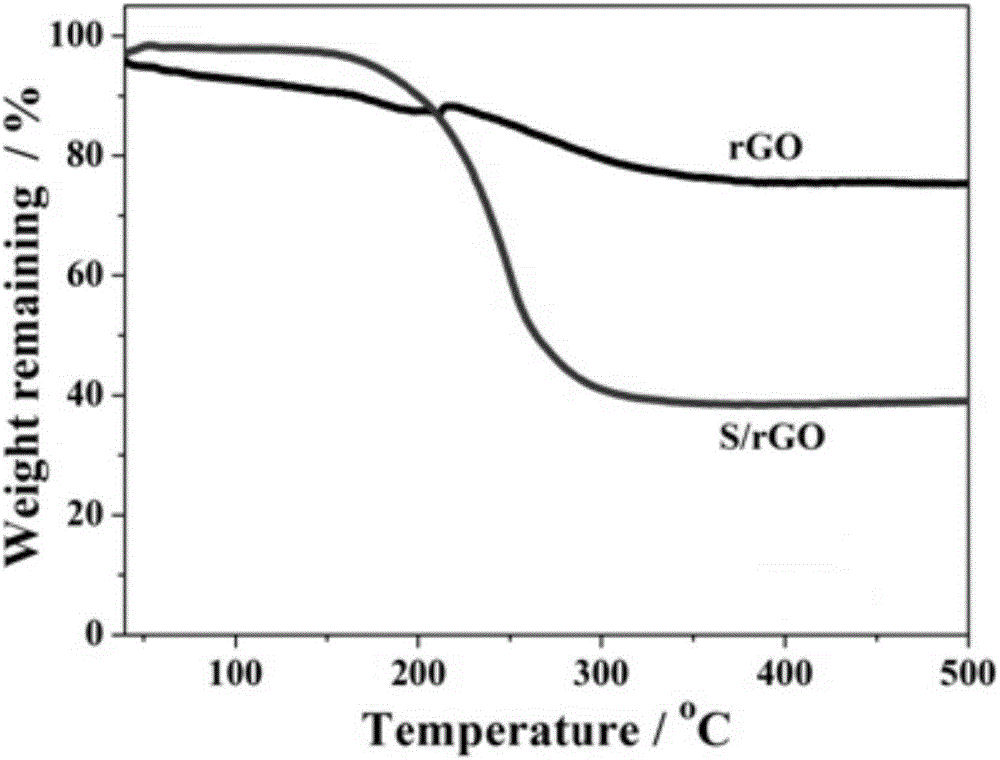
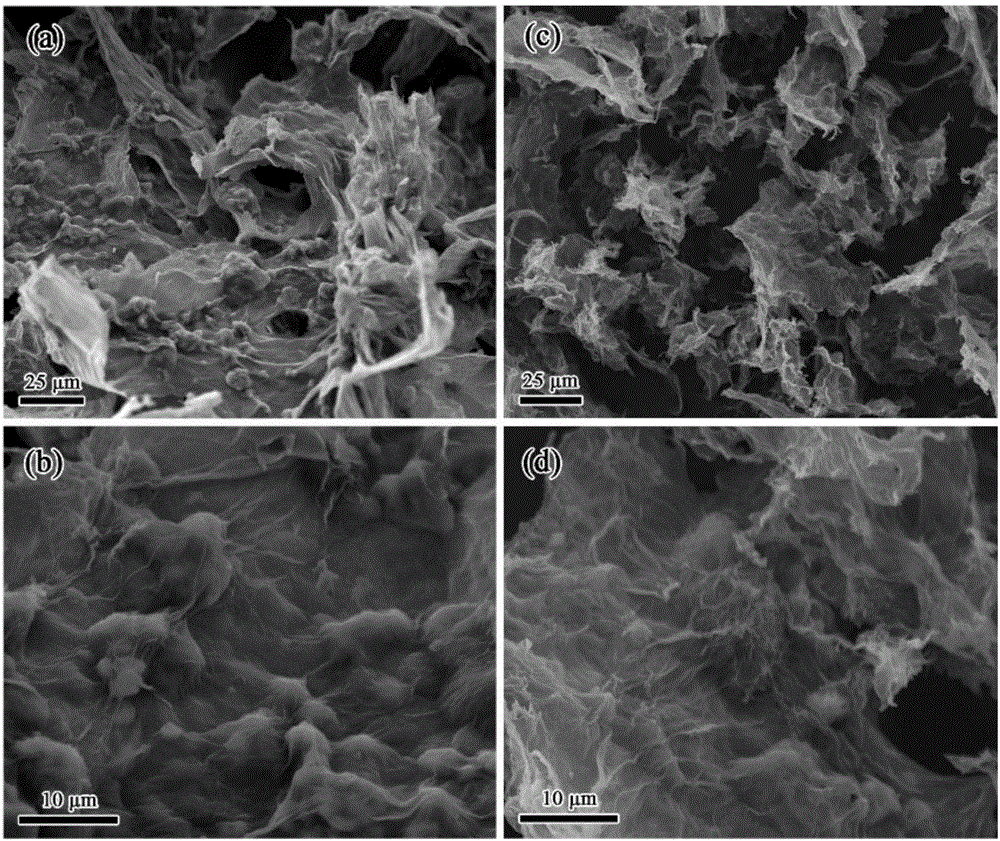
Three-dimensional graphite aerogel sulfur-carrying composite material as well as preparation method and application thereof
A technology of graphene airgel and composite materials, which is applied in the field of electrode materials for lithium-sulfur batteries, can solve problems such as poor cycle performance and rate performance, unsatisfactory material conductivity, complicated preparation process, etc., and achieve convenient large-scale production , improve cycle stability and rate performance, and alleviate the effect of volume change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of three-dimensional graphene oxide airgel sulfur-loaded (S / GO) composites: Disperse 50 mg of graphene oxide and 50 mg of sublimed sulfur in 5 mL of distilled water, add 0.5 mL of 10 mg mL at room temperature (25 °C) -1 Chitosan (molecular weight is 150,000) / acetic acid aqueous solution (volume ratio of acetic acid to water is 5:95), stirred until hydrogel is formed, then left to stand at room temperature for 6 hours, and freeze-dried to obtain three-dimensional graphite oxide Sulfur-loaded (S / GO) composites on olefin airgel.
[0034] (2) Preparation of three-dimensional graphene airgel sulfur-loaded (S / rGO) composite material: disperse 105 mg of the product obtained in step (1) with 5 mL of distilled water, add 0.3 mL of ammonia (25 wt %) and 0.2mL hydrazine hydrate (50wt%) was reacted for 1.5 hours, suction filtered and washed with distilled water until the filtrate was neutral, and the collected solid was freeze-dried to obtain the three-dimensional g...
Embodiment 2
[0042] (1) Preparation of three-dimensional graphene oxide airgel sulfur-loaded (S / GO) composites: Disperse 25 mg of graphene oxide and 50 mg of sublimed sulfur in 5 mL of distilled water, add 0.5 mL of 10 mg mL at room temperature (25 °C) -1 Chitosan (molecular weight is 150,000) / acetic acid aqueous solution (volume ratio of acetic acid to water is 5:95), stirred until hydrogel is formed, then left to stand at room temperature for 6 hours, and freeze-dried to obtain three-dimensional graphite oxide Sulfur-loaded (S / GO) composites on olefin airgel.
[0043] (2) Preparation of three-dimensional graphene airgel sulfur-loaded (S / rGO) composite material: disperse 80 mg of the product obtained in step (1) with 5 mL of distilled water, add 0.3 mL of ammonia (30 wt %) and 0.2mL hydrazine hydrate (40wt%) was reacted for 2 hours, suction filtered and washed with distilled water until the filtrate was neutral, and the collected solid was freeze-dried to obtain the three-dimensional grap...
Embodiment 3
[0046] (1) Preparation of three-dimensional graphene oxide airgel sulfur-loaded (S / GO) composites: Disperse 50 mg of graphene oxide and 100 mg of sublimed sulfur in 5 mL of distilled water, add 0.5 mL of 10 mg mL at room temperature (25 °C) -1 Chitosan (molecular weight is 150,000) / acetic acid aqueous solution (volume ratio of acetic acid to water is 5:95), stirred until hydrogel is formed, then left to stand at room temperature for 6 hours, and freeze-dried to obtain three-dimensional graphite oxide Sulfur-loaded (S / GO) composites on olefin airgel.
[0047] (2) Preparation of three-dimensional graphene airgel sulfur-loaded (S / rGO) composite material: disperse 155 mg of the product obtained in step (1) with 10 mL of distilled water, add 0.3 mL of ammonia (15 wt %) and 0.2mL hydrazine hydrate (50wt%) was reacted for 1.2 hours, suction filtered and washed with distilled water until the filtrate was neutral, and the collected solid was freeze-dried to obtain the three-dimensional...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com