Benazepril hydrochloride tablets and preparation method thereof
A benazepril hydrochloride tablet, a technology of benazepril hydrochloride, applied in the direction of pharmaceutical formula, medical preparation of non-active ingredients, pill delivery, etc., can solve the storage stability of benazepril hydrochloride tablet, etc. Achieve the effects of ensuring safe use and long-term storage, improving stability, and strong product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
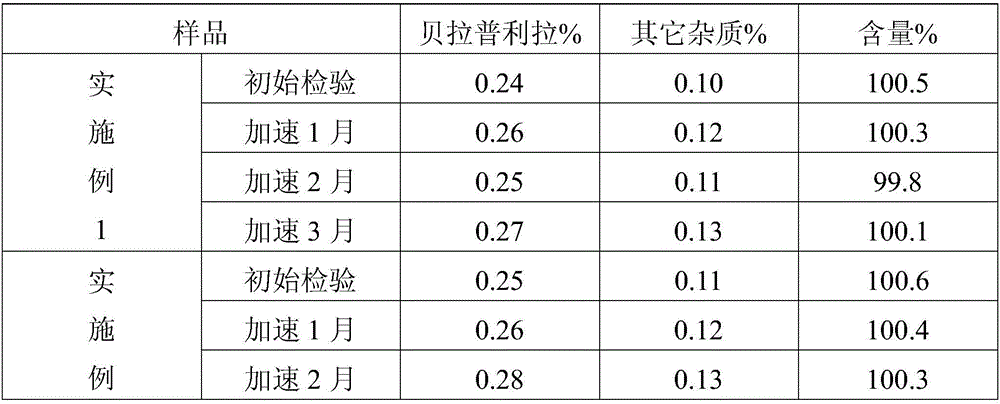
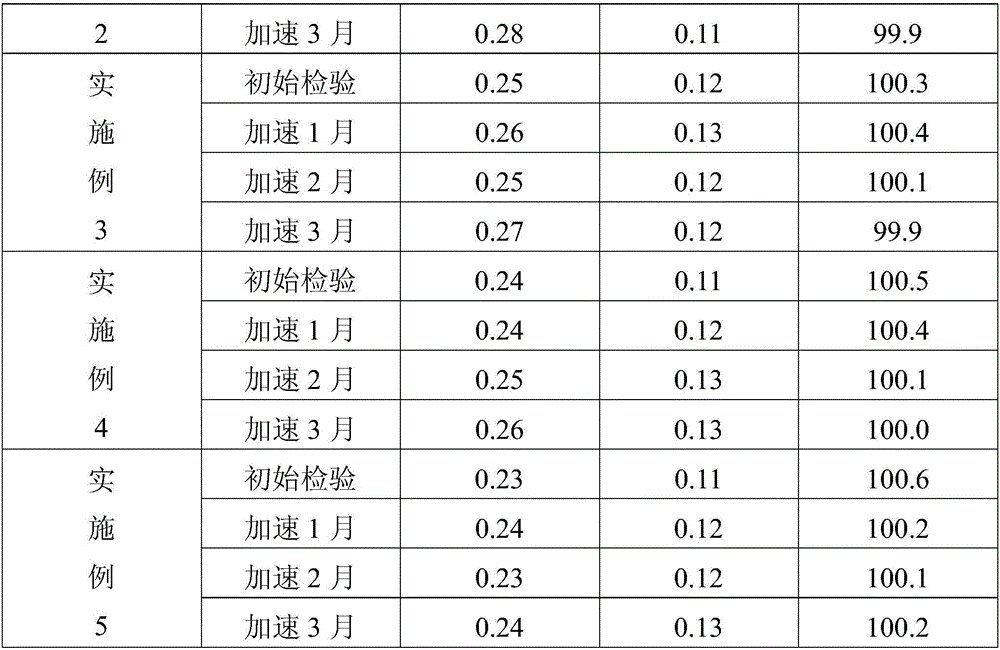
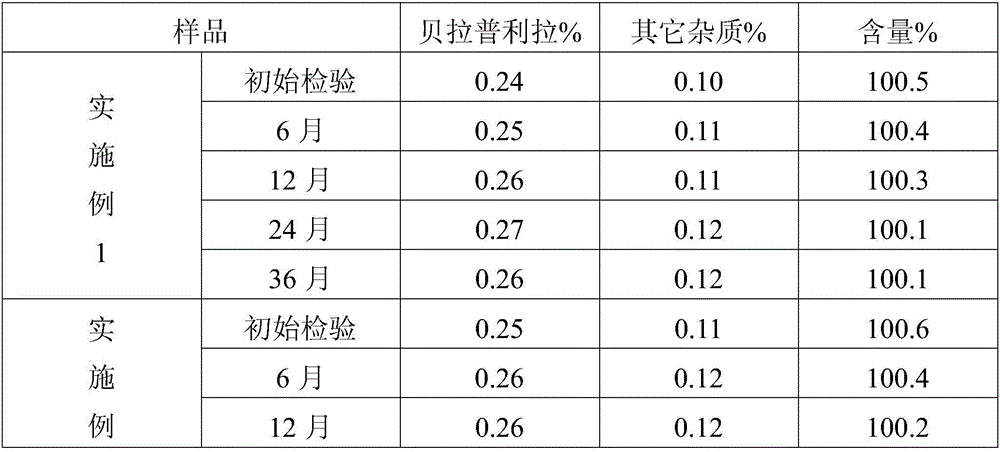
Embodiment 1
[0023] This embodiment provides a kind of benazepril hydrochloride tablet, is made of the following components by weight: benazepril hydrochloride 1.02kg, β-cyclodextrin 4kg, microcrystalline cellulose 12kg, cornstarch 1.3kg , magnesium stearate 0.2kg.
[0024] The preparation method of above-mentioned benazepril hydrochloride sheet, comprises the following steps:
[0025] (1) Weigh benazepril hydrochloride, β-cyclodextrin, microcrystalline cellulose, cornstarch and magnesium stearate according to the prescription quantity and pass through a 100-mesh sieve respectively for subsequent use;
[0026] (2) Add benazepril hydrochloride into a saturated cyclodextrin aqueous solution, stir and mix for 30 minutes, filter, dry under reduced pressure, and control the water content to 1.5%;
[0027] (3) The cyclodextrin inclusion compound of benazepril hydrochloride is pulverized and passed through an 80-mesh sieve;
[0028] (4) The cyclodextrin inclusion compound of benazepril hydrochl...
Embodiment 2
[0031] This embodiment provides a kind of benazepril hydrochloride tablet, is made of the following components by weight: benazepril hydrochloride 1.02 parts, β-cyclodextrin 3kg, microcrystalline cellulose 15kg, cornstarch 0.6kg , magnesium stearate 0.3kg.
[0032] The preparation method of above-mentioned benazepril hydrochloride sheet, comprises the following steps:
[0033] (1) Weigh benazepril hydrochloride, β-cyclodextrin, microcrystalline cellulose, cornstarch and magnesium stearate according to the prescription quantity and pass through a 100-mesh sieve respectively for subsequent use;
[0034] (2) Add benazepril hydrochloride into a saturated cyclodextrin aqueous solution, stir and mix for 30 minutes, filter, dry under reduced pressure, and control the water content to 1.7%;
[0035] (3) The cyclodextrin inclusion compound of benazepril hydrochloride is pulverized and passed through an 80-mesh sieve;
[0036] (4) The cyclodextrin inclusion compound of benazepril hydr...
Embodiment 3
[0039] This embodiment provides a Benazepril hydrochloride tablet, which is made of the following components by weight: 1.02 parts of Benazepril hydrochloride, 5 kg of β-cyclodextrin, 10 kg of microcrystalline cellulose, and 1.8 kg of corn starch , magnesium stearate 0.1kg.
[0040] The preparation method of above-mentioned benazepril hydrochloride sheet, comprises the following steps:
[0041] (1) Weigh benazepril hydrochloride, β-cyclodextrin, microcrystalline cellulose, cornstarch and magnesium stearate according to the prescription quantity and pass through a 100-mesh sieve respectively for subsequent use;
[0042] (2) Add benazepril hydrochloride into a saturated cyclodextrin aqueous solution, stir and mix for 30 minutes, filter, dry under reduced pressure, and control the water content to 1.4%;
[0043] (3) The cyclodextrin inclusion compound of benazepril hydrochloride is pulverized and passed through an 80-mesh sieve;
[0044] (4) The cyclodextrin inclusion compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com