Method for preparing tobacco mosaic virus capsid protein capsule
A technology of tobacco mosaic virus and capsid protein, which is applied in the field of biomedicine, can solve the problem of no tobacco mosaic virus capsid protein, etc., and achieve the effect of strong capsule structure, simple preparation method and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 A kind of preparation method of tobacco mosaic virus capsid protein capsule
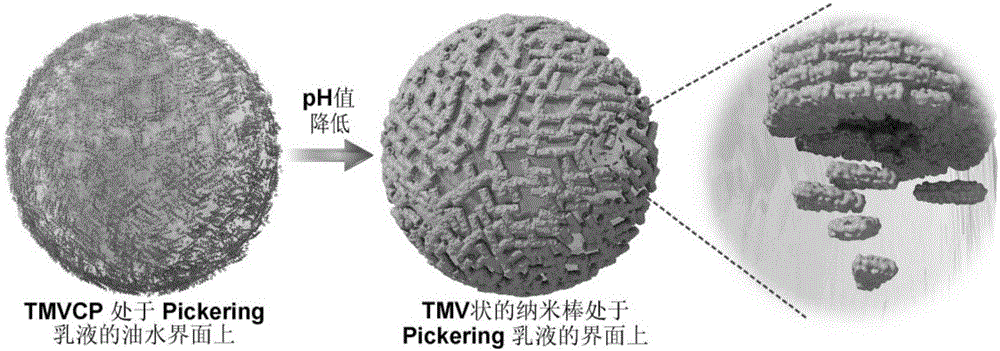
[0038] A kind of preparation method of tobacco mosaic virus capsid protein capsule comprises the following steps (its flow chart is as follows figure 1 shown):
[0039] (1) Preparation of tobacco mosaic virus capsid protein solution
[0040] Take 0.6ml of tobacco mosaic virus solution with a concentration of 10mg / ml, add 1.2ml of acetic acid to it, invert the centrifuge tube 5 times to mix well, and then let it stand in a refrigerator at 4°C for 30min. Centrifuge at 9500 rpm for 10 min at 4°C to remove the RNA pellet. The supernatant was separated through a desalting column to remove the acetic acid fraction. The resulting solution was dialyzed against deionized water for 18 h, after which it was centrifuged at 9000 rpm for 10 min at 4°C. The obtained precipitate was redissolved by adding 100 mM phosphate buffer solution with pH=8.0 to obtain a tobacco mosaic virus capsid prot...
Embodiment 2
[0045] Embodiment 2 A kind of preparation method of tobacco mosaic virus capsid protein capsule
[0046] A preparation method of tobacco mosaic virus capsid protein capsule, comprising the following steps:
[0047] (1) Preparation of tobacco mosaic virus capsid protein solution
[0048]Take 0.6ml of tobacco mosaic virus solution at a concentration of 20mg / ml, add 1.2ml of acetic acid to it, invert the centrifuge tube 5 times to mix well, and then let it stand in a refrigerator at 4°C for 40min. Centrifuge at 10,000 rpm for 8 min at 4°C to remove the RNA pellet. The supernatant was separated through a desalting column to remove the acetic acid fraction. The resulting solution was dialyzed against deionized water for 12 h and then centrifuged at 8000 rpm for 12 min at 4°C. The obtained precipitate was redissolved by adding 200 mM phosphate buffer solution with pH=8.0 to obtain a tobacco mosaic virus capsid protein solution.
[0049] (2) Preparation of Tobacco Mosaic Virus Ca...
Embodiment 3
[0053] Embodiment 3 A kind of preparation method of tobacco mosaic virus capsid protein capsule
[0054] A preparation method of tobacco mosaic virus capsid protein capsule, comprising the following steps:
[0055] (1) Preparation of tobacco mosaic virus capsid protein solution
[0056] Take 0.6ml of tobacco mosaic virus solution with a concentration of 5mg / ml, add 1.2ml of acetic acid to it, invert the centrifuge tube 5 times to mix well, and then let it stand in a refrigerator at 4°C for 30min. Centrifuge at 8000 rpm for 10 min at 4°C to remove the RNA pellet. The supernatant was separated through a desalting column to remove the acetic acid fraction. The resulting solution was dialyzed against deionized water for 24 h and then centrifuged at 9000 rpm for 10 min at 4°C. The obtained precipitate was redissolved by adding 20 mM phosphate buffer solution with pH=8.0 to obtain a tobacco mosaic virus capsid protein solution.
[0057] (2) Preparation of Tobacco Mosaic Virus Ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com