Complex and preparation method thereof as well as hydrogel
A complex and hydrogel technology, applied in the field of its preparation, complex and hydrogel, can solve problems such as large side effects, psychological and physiological damage to patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Correspondingly, the present invention provides a kind of preparation method of complex, comprises the following steps:
[0049] The drug reacts with the polymer having the structure of formula (I) or formula (II) in an aqueous medium to form a complex;
[0050]
[0051] where the R 1 Independently selected from -CH 2 -, -(CH 2 ) 2 ; 2 independently selected from H ions, metal cations or organic cations;
[0052] m is the degree of polymerization, 10≤m≤227, preferably 20≤m≤185, more preferably 30≤m≤115;
[0053] n is the degree of polymerization, 10≤n≤226; preferably 20≤n≤180, more preferably 30≤n≤112;
[0054] x is the degree of polymerization, 1≤x≤100; preferably 5≤x≤80; more preferably 3≤x≤50;
[0055] y is the degree of polymerization, 1≤y≤50; preferably 2≤y≤40; more preferably 3≤y≤30;
[0056] p is the degree of polymerization, 2≤p≤50; preferably 2≤p≤40; more preferably 3≤p≤30;
[0057] q is the degree of polymerization, 2≤q≤50; preferably 2≤q≤40; more ...
Embodiment 1
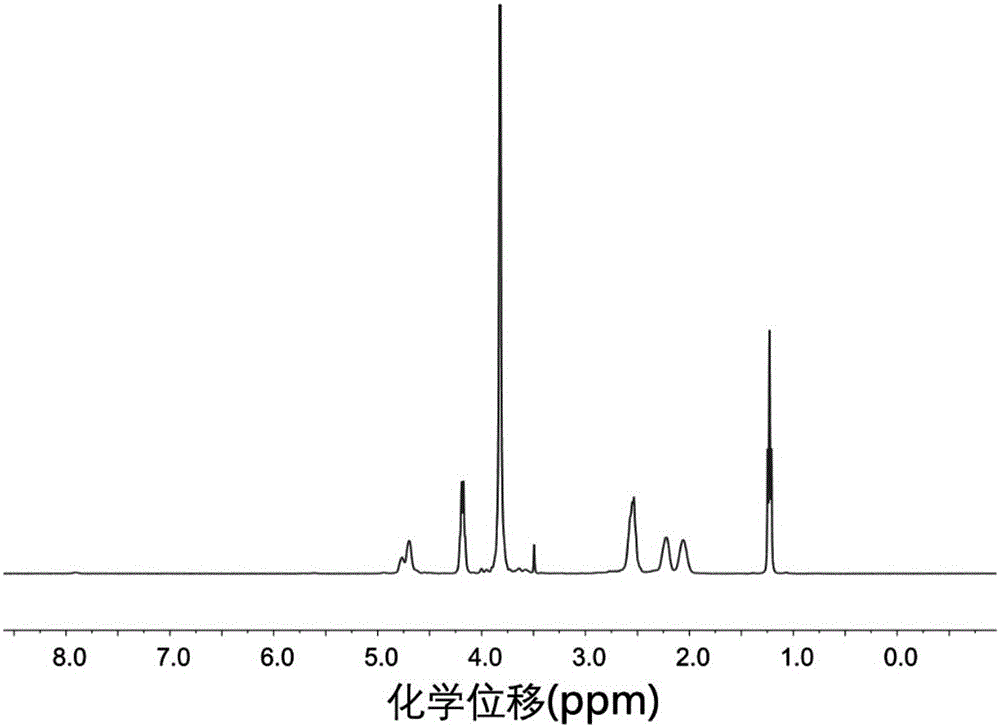
[0126] Azeotrope 6.0 g of aminated polyethylene glycol monomethyl ether with a number average molecular weight of 2000 and 200 mL of toluene at 130 ° C for 3 h to remove water, then vacuum the remaining toluene to dryness; dissolve the obtained solid in 100 mL In dried N,N-dimethylformamide, the first solution was obtained; 9.0 g of γ-ethyl-L-glutamic acid ester-N-carboxylic acid internal anhydride and 4.75 g of γ-benzyl-L-glutamine Amino acid ester-N-carboxylic acid internal acid anhydride was dissolved in 160mL of dry N,N-dimethylformamide to obtain a second solution; in a nitrogen atmosphere, the first solution was mixed with the second solution, at room temperature , stirring and reacting for 80 hours under the protection of nitrogen; after the reaction, drain N,N-dimethylformamide under reduced pressure, then dissolve the obtained solid in chloroform, settle with ether, filter with suction, and dry to obtain Polyethylene glycol monomethyl ether-polyamino acid ester block ...
Embodiment 2
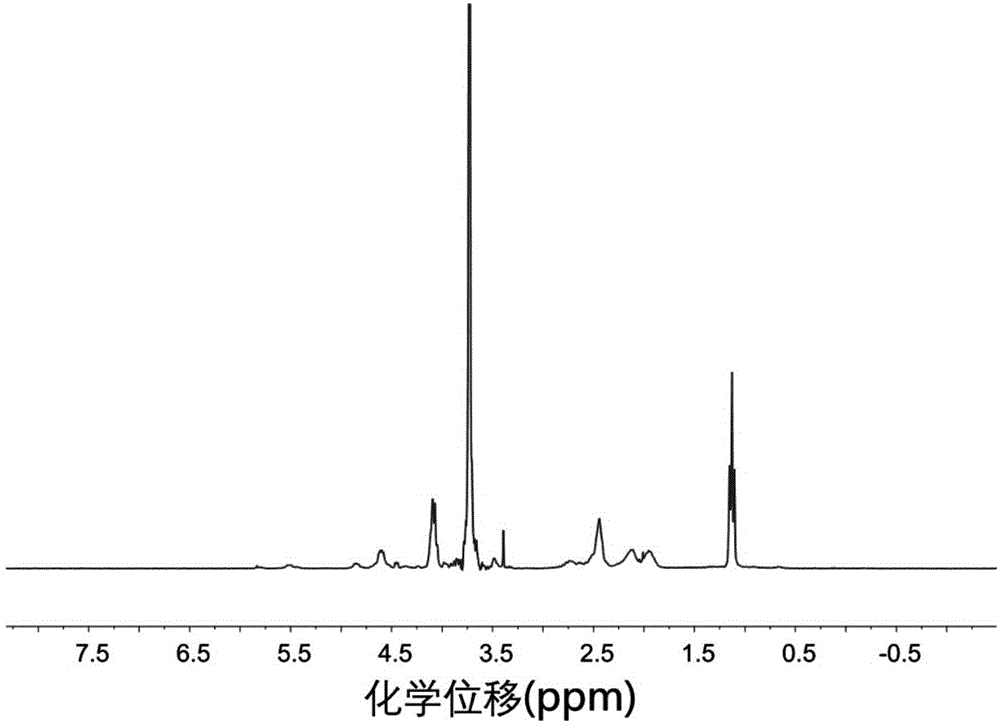
[0129] Azeotrope 3.1 g of aminated polyethylene glycol monomethyl ether with a number average molecular weight of 2000 and 100 mL of toluene at 120 ° C for 3 h to remove water, and then drain the remaining toluene under reduced pressure; dissolve the obtained solid in 30 mL In dried N,N-dimethylformamide, the first solution was obtained; 4.7g of γ-ethyl-L-glutamic acid ester-N-carboxylate and 1.2g of γ-benzyl-L-glutamine Amino acid ester-N-carboxylic acid internal acid anhydride was dissolved in 62mL of dry N,N-dimethylformamide to obtain a second solution; in a nitrogen atmosphere, the first solution was mixed with the second solution, at room temperature , stirring and reacting for 70 hours under nitrogen protection; after the reaction, drain N,N-dimethylformamide under reduced pressure, then dissolve the obtained solid in chloroform, settle with ether, filter with suction, and dry to obtain Polyethylene glycol monomethyl ether-polyamino acid ester block polymer. Undeprotec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com