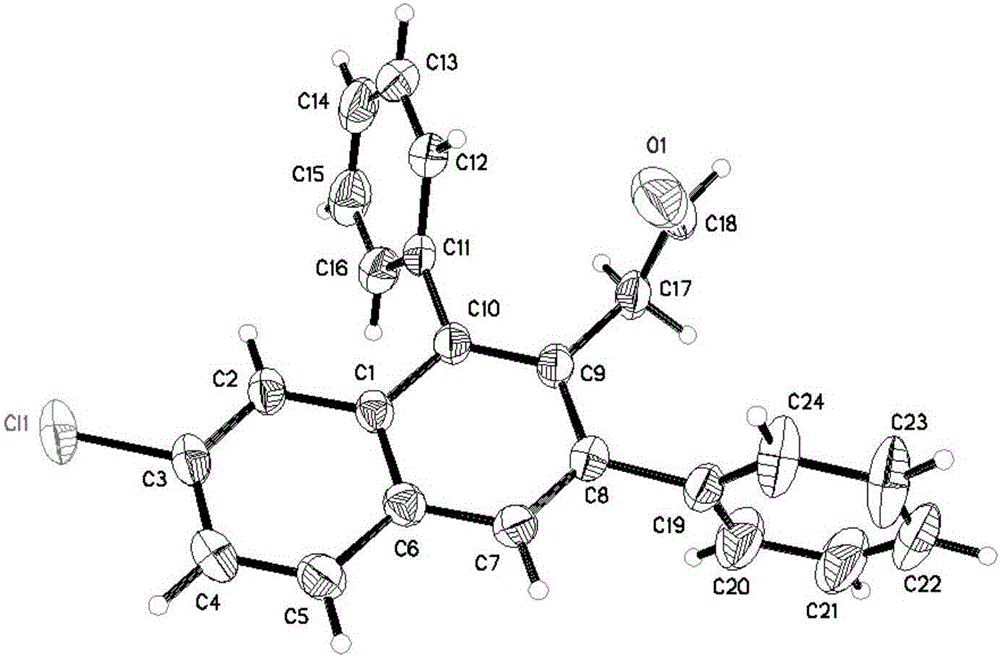
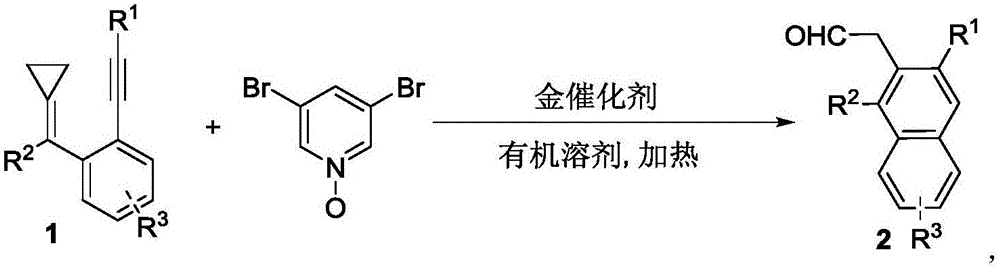
3-aryl-2-naphthalene acetaldehyde and 3-aryl-2-anthracene acetaldehyde compound as well as synthetic method and application thereof
A synthesis method and technology of naphthaleneacetaldehyde, applied in the oxidation preparation of carbonyl compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of inconvenient operation of arylacetaldehyde and difficulty in the synthesis of metal complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 --- the general operating procedure of synthetic compound 2
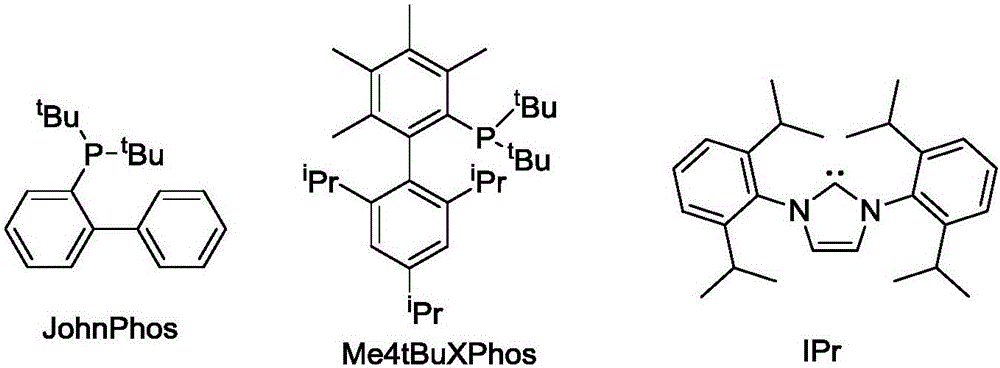
[0052] Synthesis of 3-aryl-2-naphthylacetaldehyde 2: In a clean reaction tube filled with argon, add methylenecyclopropane-substituted 1,5-enyne (1, 0.20 mmol), 3,5-dibromo Pyridine oxynitride (0.3mmol), gold catalyst JohnPhosAu(NCMe)SbF 6 (0.01 mmol), heated to 95°C in toluene for 4 hours. Flash column chromatography (SiO 2 , the eluent is petroleum ether / ethyl acetate=25:1) to obtain the corresponding product 2.
[0053] Compound 2a(R 1 =Ph,R 2 =Ph,R 3 =H): 59mg, 92%, white solid, melting point: 143-145°C; IR(CH 2 Cl 2 ):ν3055,2954,2923,2826,1722,1488,1442,1382,1109,1028,892,751,703cm -1 ; 1 HNMR (400MHz, CDCl 3 ,TMS):δ3.63(s,2H),7.26(d,2H,J=7.2Hz),7.33-7.40(m,4H),7.42-7.49(m,7H),7.80(s,1H), 7.85(d,1H,J=8.4Hz),9.41(s,1H); 13 C NMR (100MHz, CDCl 3 MS( 323.1(M+H + ,100); HRMS (ESI) calculated value Calcd.forC 24 h 19 o + Measured value requires: 323.1430, Found: 323.1442.
[00...
Embodiment 2
[0079] The synthesis of embodiment 2 compound 3r
[0080] Synthesis of polycyclic aromatic hydrocarbons benzo[α]anthracene 3r: In a 25mL clean reaction tube, add 3-aryl-2-naphthylacetaldehyde (2, 0.20mmol), Lewis acid In(OTf) 3 (0.01 mmol), heated to 50° C. in DCE (dichloroethane) for 2 hours. Flash column chromatography (SiO 2 , the eluent is petroleum ether) to obtain the corresponding product 3r.
[0081]
[0082] Compound 3r: 69mg, 90%, white solid, melting point: 185-187℃; IR(CH 2 Cl 2 ):ν2958,2920,2851,1654,1633,1469,1075,1019,873,822,742,700cm -1 ; 1 H NMR (400MHz, CD 2 Cl 2 ,TMS):δ7.28(d,2H,J=8.4Hz),7.40-7.44(m,2H),7.49(d,1H,J=8.8Hz),7.53(td,1H,J 1 =8.0Hz,J 2 =1.2Hz),7.58-7.62(m,2H),7.66-7.71(m,3H),7.79(d,1H,J=7.6Hz),8.14(d,1H,J=8.0Hz),8.86(d ,1H,J=8.0Hz),9.23(s,1H); 13 C NMR (100MHz, CD 2 Cl 2 ,TMS):δ121.7,121.9,123.0,125.1,125.6,126.0,126.3,127.0,127.19,127.23,128.47,128.52,128.53,128.7,130.3,130.6,131.39,131.42,135.9 (MS ESI)m / z:383.0(M+H + ,100); ...
Embodiment 3
[0083] The synthesis of embodiment 3 compound 4r
[0084] Synthesis of anticancer drug 4r: In a 25mL clean reaction tube filled with argon, add polycyclic aromatic hydrocarbon 3r (0.2mmol), catalyst Pd 2 (dba) 3 (0.01mmol), ligand XantPhos (0.02mmol), base Cs 2 CO 3 (0.4mmol), morpholine (0.4mmol), heated to 100°C in toluene, reacted for 4 hours. Flash column chromatography (SiO 2 , the eluent is petroleum ether / ethyl acetate=10 / 1) to obtain the corresponding product 4r.
[0085]
[0086] Compound 4r: 49mg, 63%, white solid, melting point: 218-220℃; IR(CH 2 Cl 2 ):ν2962,2919,2848,2820,1645,1607,1515,1502,1446,1257,1234,1122,928,831,822,803,770,751cm -1 ; 1 H NMR (400MHz, CD 2 Cl 2 ,TMS): δ3.03(t, 4H, J=4.0Hz), 3.94(t, 4H, J=4.0Hz), 7.09(d, 2H, J=7.6Hz), 7.33(d, 2H, J= 8.4Hz), 7.42(t, 1H, J=7.6Hz), 7.54(t, 1H, J=7.6Hz), 7.58-7.62(m, 3H), 7.68(d, 1H, J=8.0Hz), 7.73 (t, 1H, J=8.4Hz), 7.81(d, 1H, J=8.0Hz), 8.15(d, 1H, J=8.4Hz), 8.89(d, 1H, J=8.4Hz), 9.23(s ,1H); 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com