Heterocyclic compound for treating osteoporosis as well as preparation method and application of heterocyclic compound
A technology of heterocyclic compounds and compounds, applied in the field of medicine, can solve problems such as efficacy and side effects that have not been fully studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
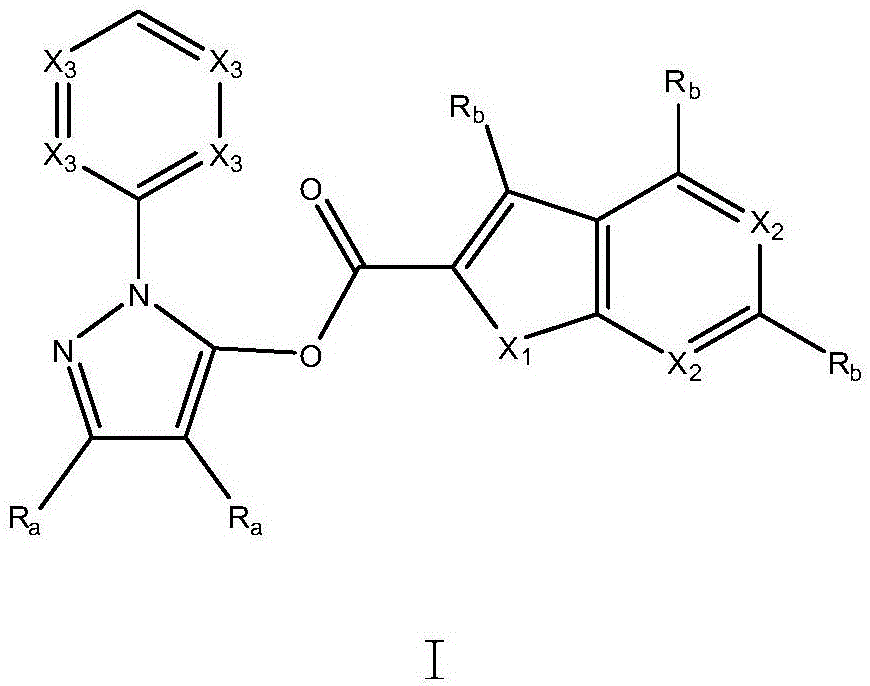
[0057] Example 1: 1-(pyridin-2-yl)-1H-pyrazol-5-ylbenzofuran-2-carboxylate (compound 1)
[0058]
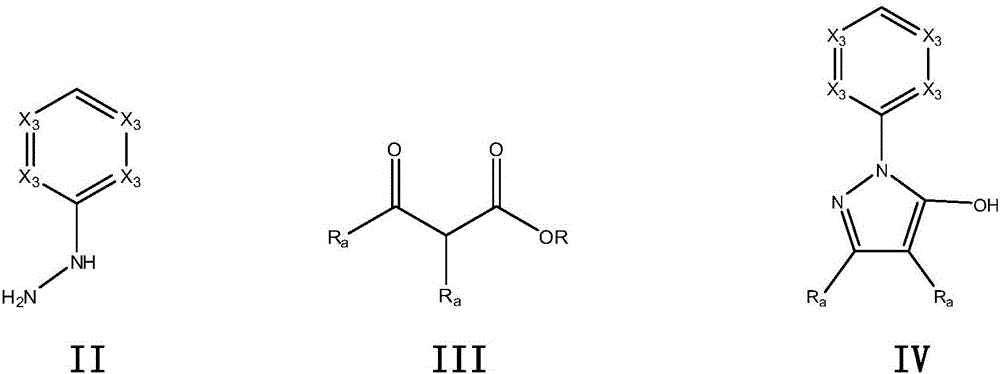
[0059] Step 1) 3-Oxo-propionic acid ethyl ester (2.32g, 20.0mmol) and ethanol (20mL) were placed in a round bottom flask, and slowly added dropwise at 0°C with 2- Hydrazinopyridine (2.58 g, 23.6 mmol). The resulting solution was heated at 100° C. for 3 days at reflux. The solvent was removed by distillation under reduced pressure, and the resulting solid was washed with hexane and ethyl acetate and dried in vacuo to obtain 2.91 g of white solid 1-(pyridin-2-yl)-1H-pyrazol-5-ol, Yield 90%.
[0060] ESI-MS: 162.06[M+H] +
[0061] Step 2) under ice bath condition, mix 2-hydroxyl-benzaldehyde (2.68g, 22.0mmol) and anhydrous K 2 CO 3 (4.4g, 31.2mmol) was dissolved in DMF (40ml), and ethyl bromoacetate (4.1g, 26.0mmol) was slowly added dropwise. After the dropwise addition was completed, the mixture was stirred at -5°C for 30 minutes, and then reacted in an oil bath at 50°C f...
Embodiment 2
[0067] Example 2: 3-methyl-4-phenyl-1-(pyridin-2-yl)-1H-pyrazol-5-ylbenzofuran-2-carboxylate (compound 2)
[0068]
[0069] Step 1) 3-oxo-2-phenyl-propionic acid ethyl ester (4.12g, 20.0mmol) and ethanol (20mL) were placed in a round bottom flask, and slowly added dropwise with ethanol (20mL ) diluted 2-hydrazinopyridine (2.58 g, 23.6 mmol). The resulting solution was heated at 100° C. for 3 days at reflux. The solvent was removed by distillation under reduced pressure and the resulting solid was washed with hexane and ethyl acetate and dried in vacuo to give 3-methyl-4-phenyl-1-(pyridin-2-yl)-1H as a white solid - 4.42 g of pyrazol-5-ol, yield 88%.
[0070] ESI-MS: 252.11[M+H] +
[0071] Step 2) under ice bath condition, mix 2-hydroxyl-benzaldehyde (2.68g, 22.0mmol) and anhydrous K 2 CO 3 (4.4g, 31.2mmol) was dissolved in DMF (40ml), and ethyl bromoacetate (4.1g, 26.0mmol) was slowly added dropwise. After the dropwise addition was completed, the mixture was stirred ...
Embodiment 3
[0077] Example 3: 3-(piperidin-1-yl)-1-(pyridin-2-yl)-1H-pyrazol-5-ylbenzothiophene-2-carboxylate (Compound 3)
[0078]
[0079]Step 1) Place 3-oxo-3-(piperidin-1-yl)-propionic acid ethyl ester (4.02g, 20.0mmol) and ethanol (20mL) in a round bottom flask, and slowly 2-Hydrazinopyridine (2.58 g, 23.6 mmol) diluted with ethanol (20 mL) was added dropwise. The resulting solution was heated at 100° C. for 3 days at reflux. The solvent was removed by distillation under reduced pressure, and the resulting solid was washed with hexane and ethyl acetate and dried in vacuo to give a white solid 3-(piperidin-1-yl)-1-(pyridin-2-yl)- 3.91 g of 1H-pyrazol-5-ol, yield 81%.
[0080] ESI-MS: 245.13[M+H] +
[0081] Step 2) under ice bath condition, mix 2-nitro-benzaldehyde (3.02g, 20mmol) and anhydrous K 2 CO 3 (4.4g, 31.2mmol) was dissolved in DMF (40ml), and ethyl thioglycolate (2.40g, 20mmol) was slowly added dropwise. After the dropwise addition was completed, the mixture was sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com