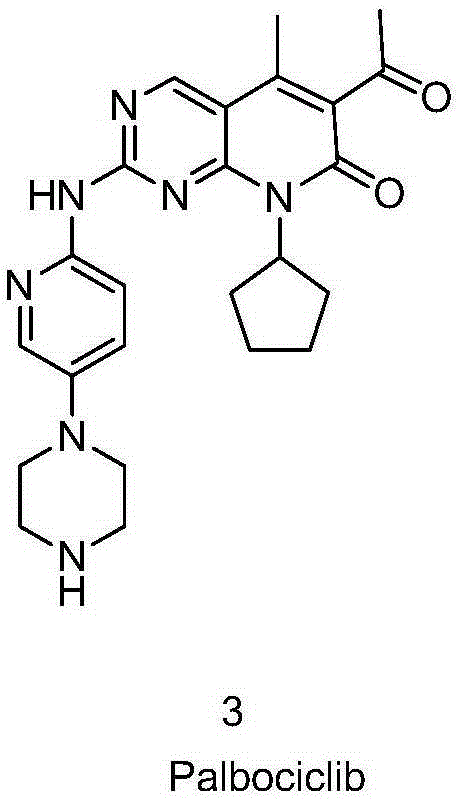
Palbociclib and preparation method of intermediate of palbociclib
A compound and equivalent technology, applied in the preparation of tert-butyl 4-{6-[amino]-3-pyridyl}-1-piperazinecarboxylate, palbociclib and its new key intermediates, The field of preparation of the drug palbociclib and its intermediates can solve the problems of difficult removal of impurities, low product purity, and difficult purification, and achieve the effects of simple operation, control of impurity content, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
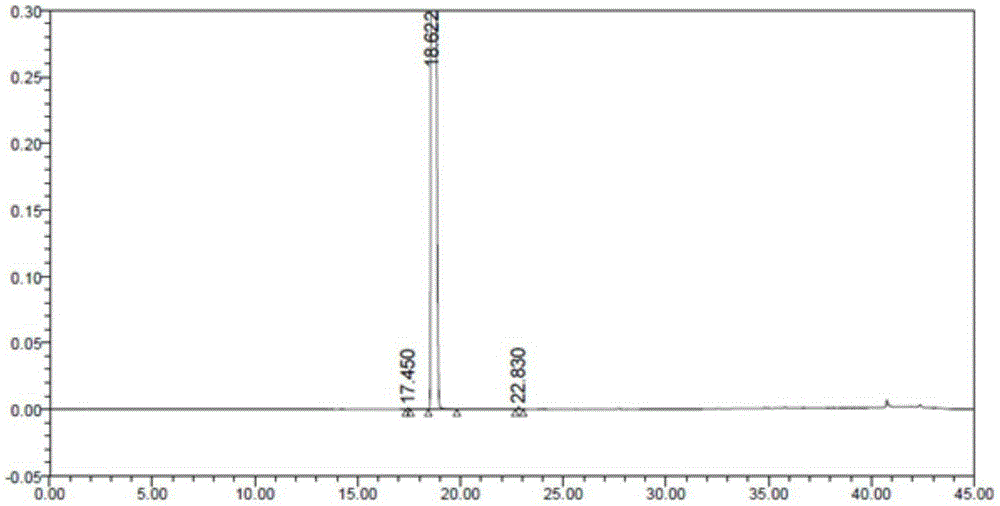
Image
Examples
Embodiment 1
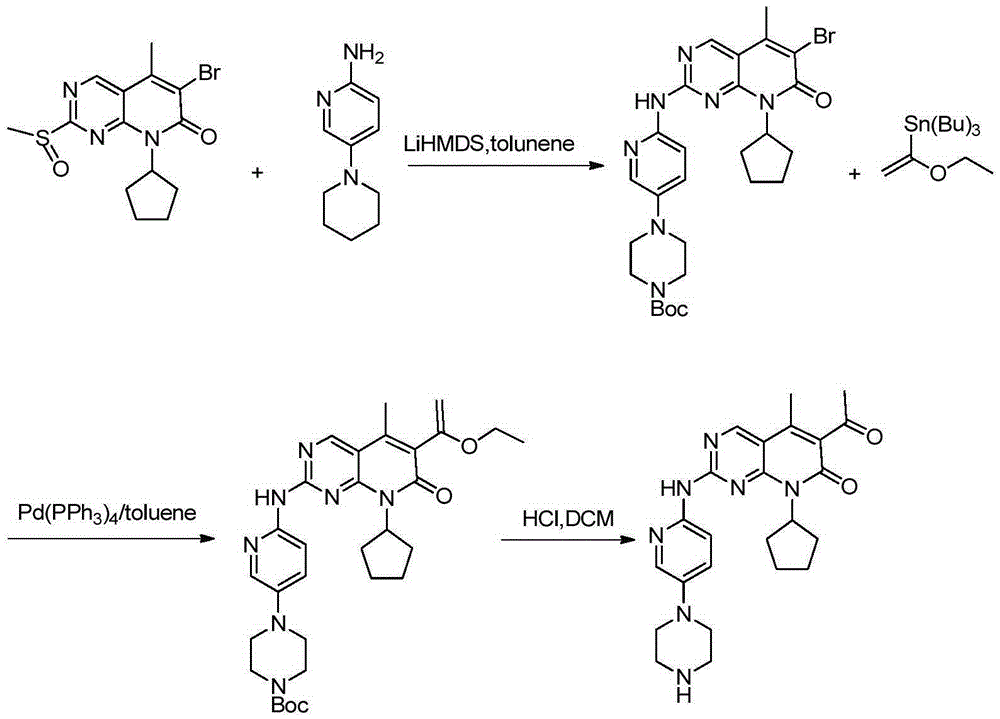
[0037] Example 1: 4-{6-[(6-(1-butoxy-vinyl)-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydropyrido[2 ,3-D]pyrimidin-2-yl)amino]-3-pyridyl}-1-piperazinecarboxylate tert-butyl ester (synthesis of compound 2) was prepared by referring to the method in patent WO2005 / 005426
[0038] Under nitrogen atmosphere, mix 4.7L n-butanol, 768gXT46-A, 275mL DIPEA and 395g vinyl butyl ether, stir nitrogen replacement 3 times, add 22g catalyst Pd(dppf)2Cl2, and nitrogen replacement 3 times again, then It was heated to 95° C. under the protection of nitrogen, and the stirring reaction was continued at this temperature for 20 hours. The resulting light red slurry was diluted with 4 L of octane, cooled to 5°C, added with 1 L of saturated potassium carbonate aqueous solution, filtered and washed with 500 mL of octane. After hours at 45°C, 664 g of off-white solid (compound 2) were obtained, yield: 83%.
[0039] It was confirmed to be compound 2 by detection.
Embodiment 2
[0040] Example 2: Intermediate 4-{6-[(6-acetyl-8-cyclopentyl-5-methyl-7-oxo-7,8-dihydropyrido[2,3-D]pyrimidine -2-yl)amino]-3-pyridyl}-1-piperazinecarboxylic acid tert-butyl ester (preparation of compound 1)
[0041] Add 50.00 mL of methanol and 3.32 g of compound 2 into a 100 mL three-necked flask, cool down to 0-10° C. in an ice-water bath, and add 20 mL of 1N phosphoric acid dropwise. After the addition was completed, the temperature was controlled at 25°C and the reaction was stirred for 16 hours. The reaction solution was filtered and rinsed with methanol. Dissolve the solid in 50 mL of methanol and heat to reflux until the solid dissolves. After stirring for another 0.5 hour, cool to room temperature, filter, and rinse with methanol. The solid was dried in a vacuum oven at 40° C. for 12 hours to obtain 2.41 g of a yellow solid (compound 1), yield: 80%.
[0042] It was confirmed to be compound 1 by detection.
[0043] 1 H-NMR (CD 3 Cl):1.49(9H,s);1.69(2H,m);1.90(2H,...
Embodiment 3
[0048] Add 50.00 mL of methanol and 3.32 g of compound 2 into a 100 mL three-necked flask, cool down to 0-10° C. in an ice-water bath, and add 35 mL of 0.5 N sulfuric acid dropwise. After the addition, the temperature was controlled to 25°C and the reaction was stirred for 16 hours. The reaction solution was filtered and rinsed with methanol. Dry in a vacuum oven at 40° C. for 12 hours to obtain 2.63 g of a yellow solid (compound 1), yield: 87%.
[0049] It was confirmed to be compound 1 by detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com