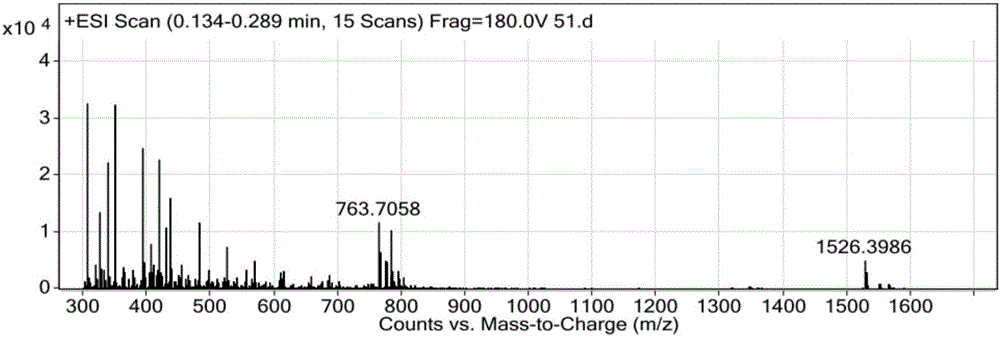
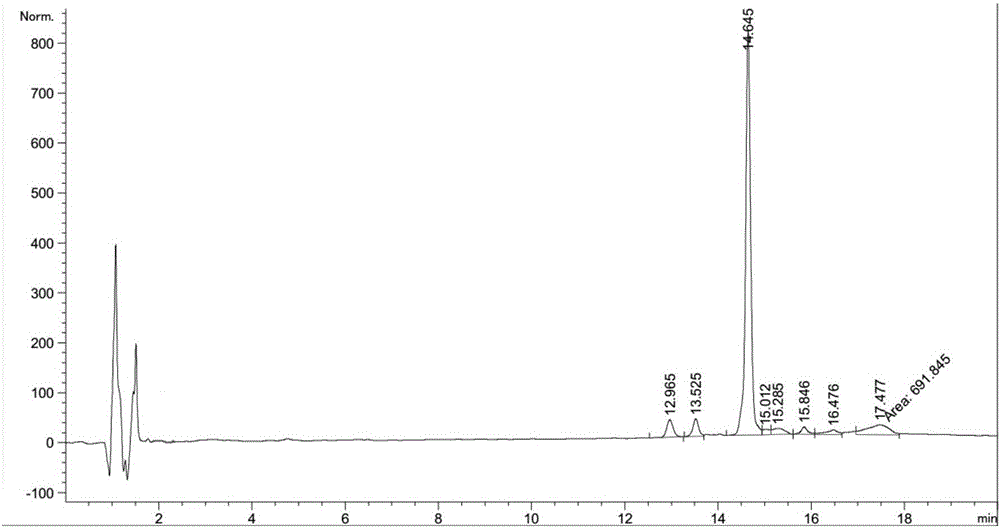
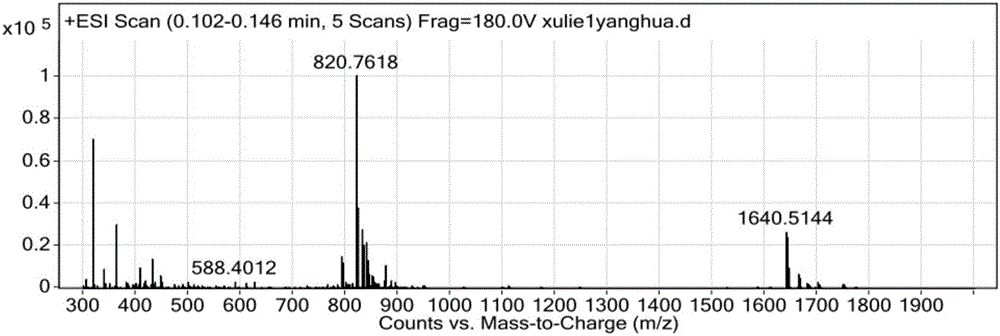
Method for preparing Linaclotide
A technology for linaclotide and crude peptides, which is applied in the production of peptides and bulk chemicals, can solve problems such as cumbersome steps, and achieve the effects of cheap and easy-to-obtain raw materials, mild reaction conditions, high reaction selectivity and conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of linaclotide resin
[0049] H-Cys(tBu)-Cys(Trt)-Glu(OtBu)-Tyr(tBu)-Cys(Trt)-Cys(tBu)-Asn(Trt)-Pro-Ala-Cys(Trt)-Thr(tBu) Preparation of -Gly-Cys(Trt)-Tyr(tBu)—Resin
[0050] Weigh 10 g of Wang resin with a substitution degree of 1.14 mmol / g in a solid-phase reactor, swell the Wang resin with DCM for 20 minutes, and drain it.
[0051] Add Fmoc-Tyr(tBu)-OH (15.7g, 13.68mmol), HOBt (5.55g, 4.1mmol), DIC (6.35mL, 4.1mmol), DMAP (0.5g, 4.1mmol) into the solid phase reactor to dissolve In DMF (70ml), react at room temperature for 15 minutes.
[0052] The resin was washed and dried to obtain Fmoc-Tyr(tBu)-Wang resin, and the measured resin substitution degree was 0.426 mmol / g. Add 70 mL of blocking reagent (acetic anhydride (mmol): DIPEA (mmol) = 1:1) to the resin, react for 10 h, block the remaining amino groups, and use DCM (once), MeOH (once) and DMF (once )washing.
[0053] Add 20% PIP / DMF solution to react for 20min to remove the Fmoc protecting group, w...
Embodiment 2
[0068] Preparation of linaclotide resin
[0069] H-Cys(Trt)-Cys(Trt)-Glu(OtBu)-Tyr(tBu)-Cys(tBu)-Cys(Trt)-Asn(Trt)-Pro-Ala-Cys(Trt)-Thr(tBu) Preparation of -Gly-Cys(tBu)-Tyr(tBu)—Resin
[0070] Weigh 10 g of Wang resin with a substitution degree of 1.14 mmol / g in a solid-phase reactor, swell the Wang resin with DCM for 20 minutes, and drain it.
[0071] Add Fmoc-Tyr(tBu)-OH (15.7g, 13.68mmol), HOBt (5.55g, 4.1mmol), DIC (6.35mL, 4.1mmol), DMAP (0.5g, 4.1mmol) into the solid phase reactor to dissolve In DMF (70ml), react at room temperature for 15 minutes.
[0072] The resin was washed and dried to obtain Fmoc-Tyr(tBu)-Wang resin, and the measured resin substitution degree was 0.426 mmol / g. Add 70 mL of blocking reagent (acetic anhydride (mmol): DIPEA (mmol) = 1:1) to the resin, react for 10 h, block the remaining amino groups, and use DCM (once), MeOH (once) and DMF (once )washing.
[0073] Add 20% PIP / DMF solution to react for 20min to remove the Fmoc protecting group, w...
Embodiment 3
[0088] Preparation of linaclotide resin
[0089] H-Cys(Trt)-Cys(tBu)-Glu(OtBu)-Tyr(tBu)-Cys(Trt)-Cys(Trt)-Asn(Trt)-Pro-Ala-Cys(tBu)-Thr(tBu) Preparation of -Gly-Cys(Trt)-Tyr(tBu)—Resin
[0090] Weigh 10 g of Wang resin with a substitution degree of 1.14 mmol / g in a solid-phase reactor, swell the Wang resin with DCM for 20 minutes, and drain it.
[0091]Add Fmoc-Tyr(tBu)-OH (15.7g, 13.68mmol), HOBt (5.55g, 4.1mmol), DIC (6.35mL, 4.1mmol), DMAP (0.5g, 4.1mmol) into the solid phase reactor to dissolve In DMF (70ml), react at room temperature for 15 minutes.
[0092] The resin was washed and dried to obtain Fmoc-Tyr(tBu)-Wang resin, and the measured resin substitution degree was 0.426 mmol / g. Add 70 mL of blocking reagent (acetic anhydride (mmol): DIPEA (mmol) = 1:1) to the resin, react for 10 h, block the remaining amino groups, and use DCM (once), MeOH (once) and DMF (once )washing.
[0093] Add 20% PIP / DMF solution to react for 20min to remove the Fmoc protecting group, wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com