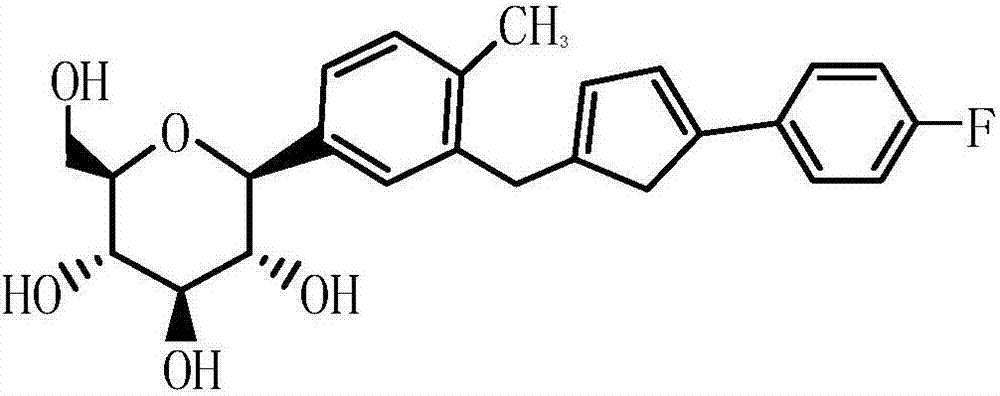
Method for continuously preparing canagliflozin by using microreactor one-pot method
A micro-reactor, canagliflozin technology, applied in the field of medicine, can solve the problems of large reaction risk factor, harsh operation requirements, hidden safety hazards, etc., achieve continuity and automation, reduce the generation of by-products, fully mixed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
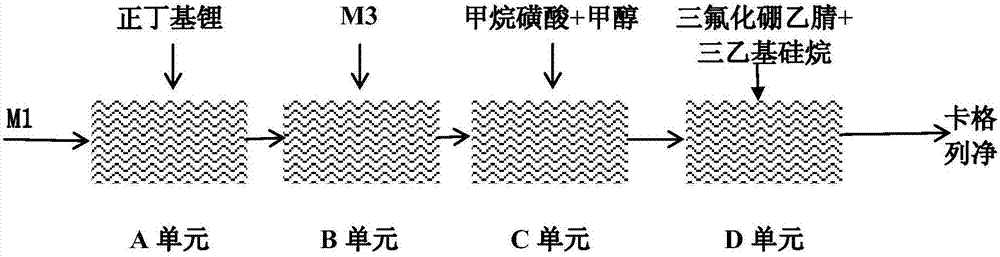
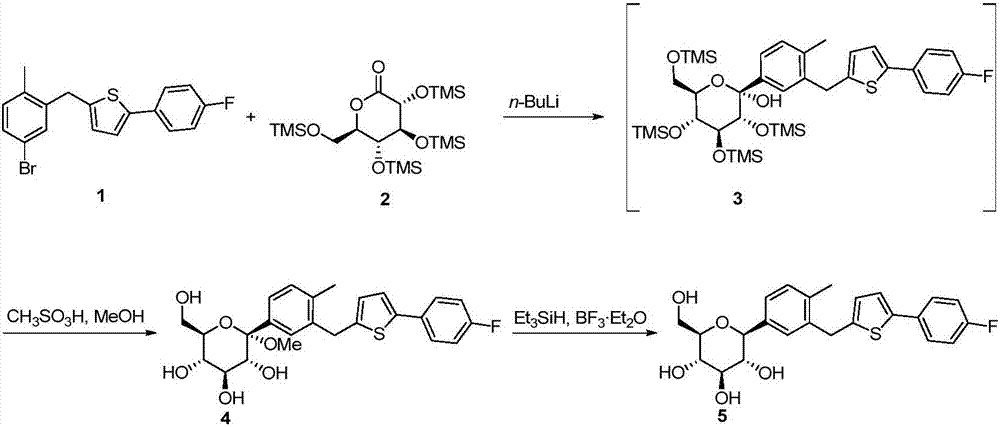
[0049] Prepare 500ml 85g / L toluene solution of the main raw material M1 in a 1L three-necked flask, flow into the A unit of the microreactor with a flow rate of 100ml / min, and add a concentration of 1.8mol / L n-butyllithium at 20ml / min The flow rate flows into unit A of the microreactor at the same time, the residence time of the reaction is 8.0 seconds, and the reaction temperature is controlled at -18°C. After the reaction liquid flows out of unit A of the microreactor, the prepared 320g / L M3 material The liquid flows into unit B of the microreactor simultaneously at a flow rate of 60 ml / min. The residence time of the reaction unit is 23.3 seconds, and the reaction temperature controlled by the reaction unit is -5°C.
[0050] Add 300ml of methanol and 100ml of methanesulfonic acid into a 500ml three-necked flask, stir and mix evenly and then cool down to room temperature. The flow rate flows into the C unit of the microreactor together. The residence time of the reaction unit...
Embodiment 2
[0053] Prepare the toluene solution of the main raw material M1 of 500ml 85g / L in the 1L three-necked flask, flow into the A unit of the microreactor with the flow rate of 110ml / min, in addition the concentration is 1.8mol / L n-butyllithium with 20ml / min The flow rate simultaneously flows into unit A, the residence time of the reaction is 7.4 seconds, and the reaction temperature is controlled at -18°C. After the reaction liquid flows out of unit A, the prepared 320g / L M3 feed liquid flows into unit B at a flow rate of 60ml / min at the same time. The residence time of the reaction unit is 22.1 seconds, and the reaction temperature controlled by the reaction unit is - 5°C.
[0054] Add 300ml of methanol and 100ml of methanesulfonic acid into a 500ml three-necked flask, stir and mix evenly and then cool down to room temperature. After the reaction feed liquid flows out of unit B, simultaneously flow the methanol solution of methanesulfonic acid into unit C at a flow rate of 20ml / m...
Embodiment 3
[0057] Prepare 500ml 85g / L toluene solution of the main raw material M1 in a 1L three-necked flask, flow it into unit A at a flow rate of 100ml / min, and simultaneously flow n-butyllithium with a concentration of 1.8mol / L into unit A at a flow rate of 20ml / min In the unit, the residence time of the reaction is 8 seconds, and the reaction temperature is controlled at -23°C. After the reaction liquid flows out of unit A, the prepared 320g / L M3 feed liquid flows into unit B at the same time at a flow rate of 60ml / min. , the residence time of the reaction unit is 23 seconds, and the reaction temperature controlled by the reaction unit is -7°C.
[0058] Add 300ml of methanol and 100ml of methanesulfonic acid into a 500ml three-necked flask, stir and mix evenly and then cool down to room temperature. After the reaction feed liquid flows out of unit B, simultaneously flow the methanol solution of methanesulfonic acid into unit C at a flow rate of 20ml / min. In the unit, the residence t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com