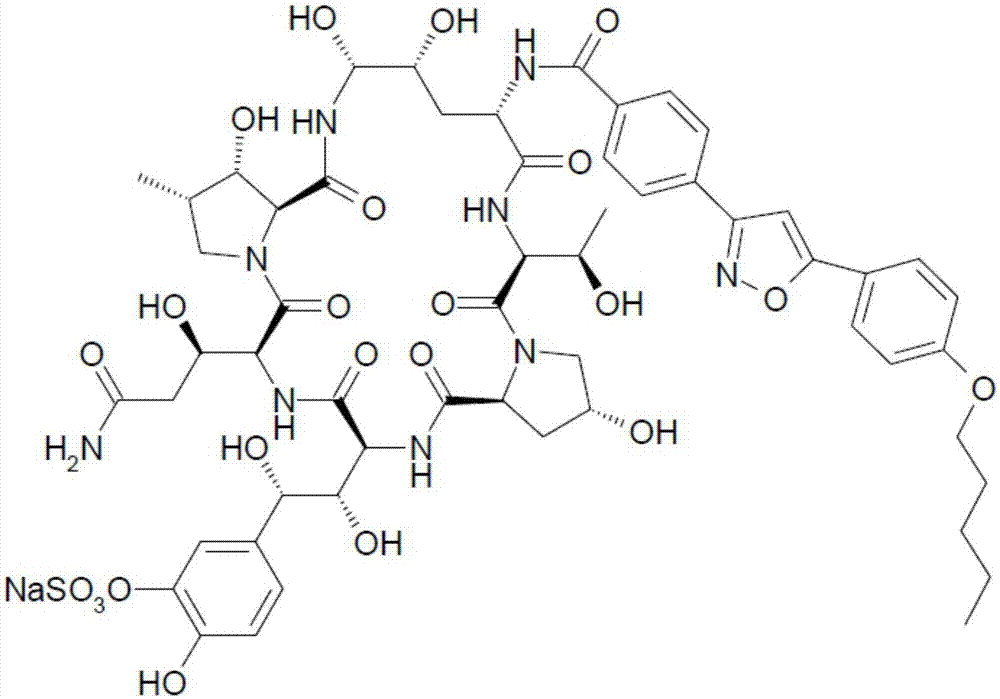
Method for synthesizing and purifying micafungin
A technology of micafungin and purification method, which is applied in the direction of organic chemistry, can solve the problems of expensive reagents, low product purity, and many waste solvents, and achieve the effect of reducing the generation of waste solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
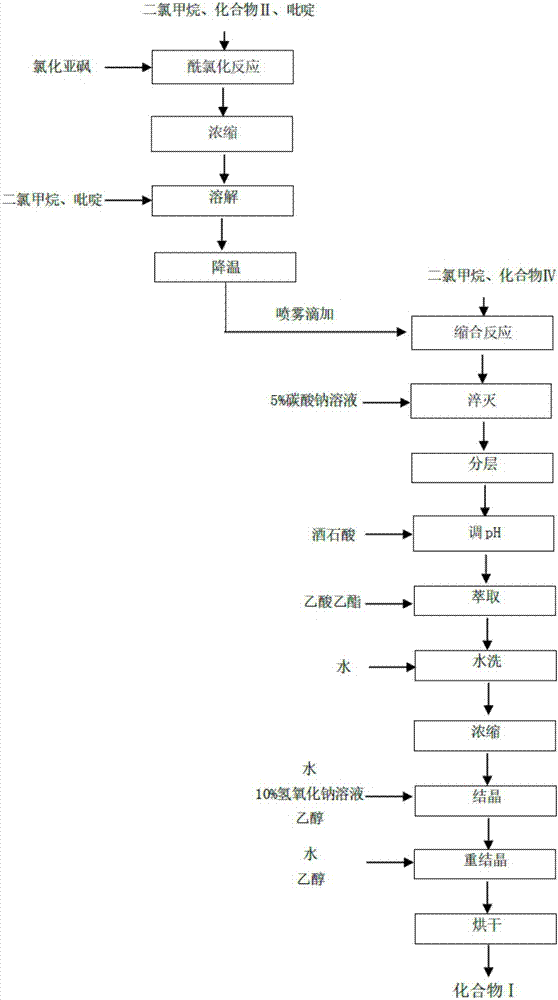
[0038] The invention provides a technical scheme: a method for synthesizing and purifying a micafungin intermediate, comprising the following steps:
[0039] Add 100ml of dichloromethane, 7.1g (20mmol, 1.0eq) of compound II and 0.2g of pyridine into a 250ml flask, stir to dissolve, raise the temperature to 22°C, add dropwise 3.0g (0.25mol, 1.25eq) of thionyl chloride, and keep for 5 After one hour, after the reaction was completed, concentrate to obtain 8.2 g of oily liquid, continue to add 100 ml of dichloromethane to dissolve, and add 4.0 g of pyridine to stir and cool down to -10°C for later use to obtain a dichloromethane solution of compound III.
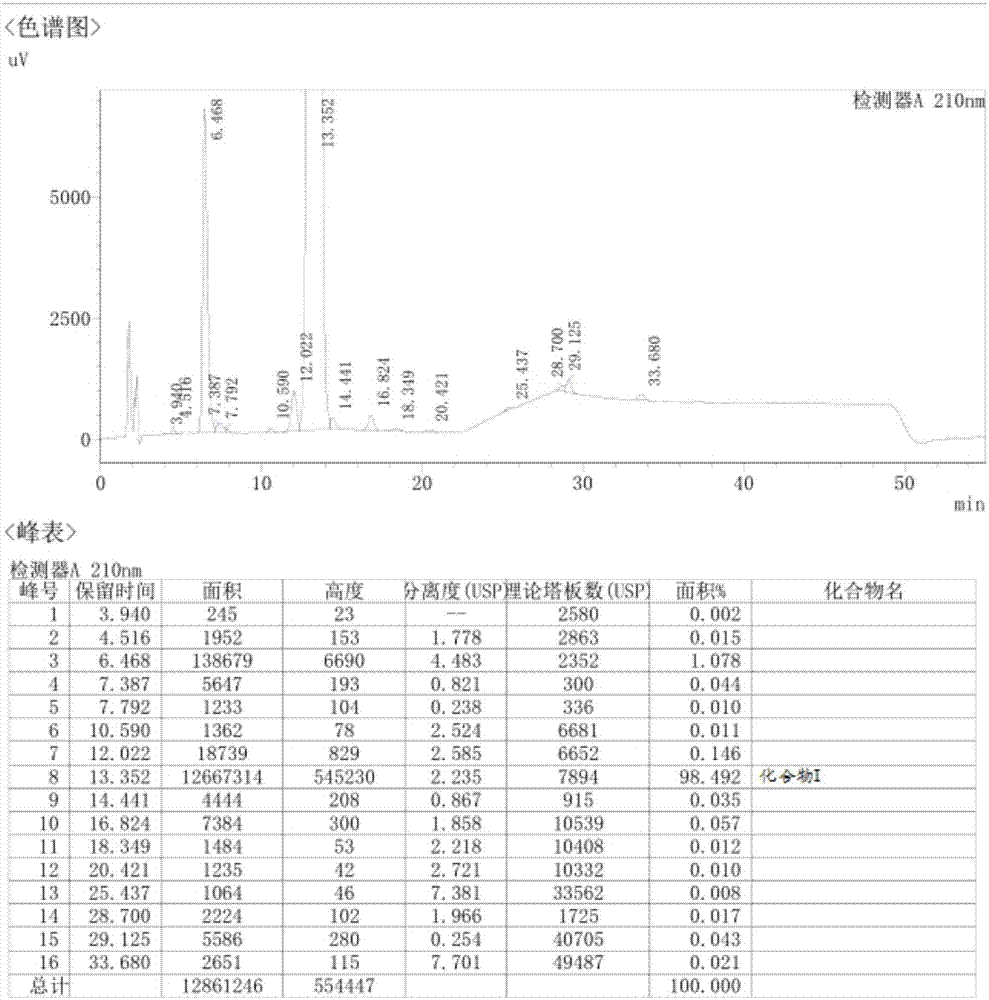
[0040] Add 100ml of dichloromethane and 18g (19.1mmol, 1.0eq) of compound IV to a 500ml flask, stir and cool down to -42°C, spray the solution of compound III dropwise, and drop it in 3.5 hours; then keep at -43°C for 8 hour; after the reaction is qualified, the reaction liquid is slowly added dropwise to 100ml of 5% sodium car...
Embodiment 2
[0045] Add 100ml of dichloromethane, 7.1g (20mmol, 1.0eq) of compound II and 0.2g of pyridine into a 250ml flask, stir to dissolve, raise the temperature to 22°C, add 3.6g (0.30mol, 1.50eq) of thionyl chloride dropwise, and keep for 5 One hour later, after the reaction was complete, concentrate to obtain 8.3 g of oily liquid, continue to add 100 ml of dichloromethane to dissolve, and add 4.5 g of pyridine to stir and cool down to -10°C for later use to obtain a dichloromethane solution of compound III.
[0046] Add 100ml of dichloromethane and 18g (19.1mmol, 1.0eq) of compound IV to a 500ml flask, stir and cool down to -45°C, spray and drop the solution of compound III for 3 hours; then keep at -42°C for 8 hour; after the reaction is qualified, the reaction liquid is slowly added dropwise to 100ml of 5% sodium carbonate solution, and stirred at room temperature for 5 hours, left to stand for stratification, and the organic phase is divided, and the aqueous phase is added dropwi...
Embodiment 3
[0050] Add 100ml of dichloromethane, 7.1g (20mmol, 1.0eq) of compound II and 0.2g of pyridine into a 250ml flask, stir to dissolve, raise the temperature to 25°C, add 3.0g (0.30mol, 1.25eq) of thionyl chloride dropwise, and keep for 5 One hour later, after the reaction was complete, concentrate to obtain 8.2 g of oily liquid, continue to add 100 ml of dichloromethane to dissolve, and add 5.0 g of pyridine to stir and cool down to -10°C for use, to obtain a dichloromethane solution of compound III.
[0051] Add 100ml of dichloromethane and 18g (19.1mmol, 1.0eq) of compound IV to a 500ml flask, stir and cool down to -45°C, spray the solution of compound III dropwise, and drop it in 4 hours; then keep at -45°C for 8 hour; after the reaction is qualified, the reaction liquid is slowly added dropwise to 100ml of 5% sodium carbonate solution, and stirred at room temperature for 5 hours, the layers are left to stand, the organic phase is separated, the aqueous phase is added dropwise ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com