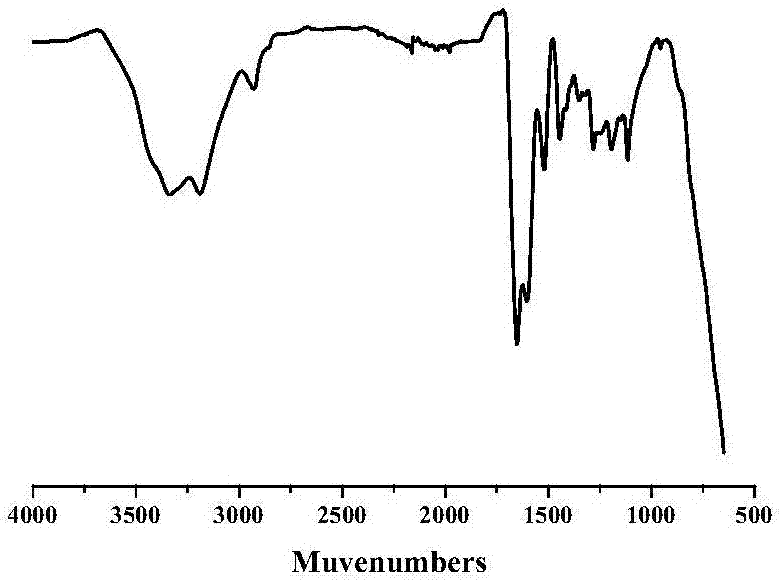
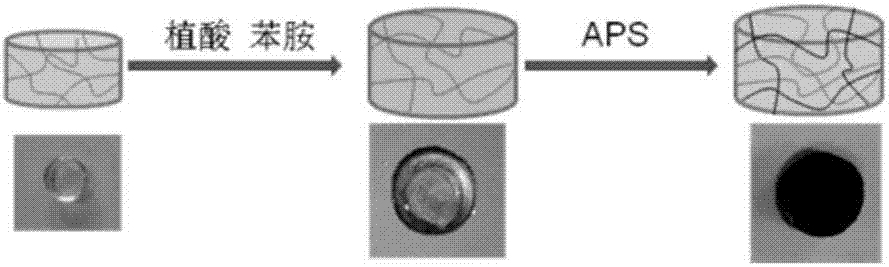
Preparation method for polyaniline-based self-repairing conductive hydrogel
A conductive hydrogel, polyaniline-based technology, which is applied in the preparation of self-healing conductive hydrogels and the preparation of polyaniline-based self-healing conductive hydrogels, can solve problems such as the influence of energy storage capacity, and achieve improved conductivity. , Excellent self-healing performance, the effect of improving self-healing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0022] Step 1: Dissolve 10g of sodium borate and 8g of sodium bicarbonate in 100mL of deionized water as the reaction medium (to protect the o-dihydroxyl group). Both sodium borate and sodium bicarbonate are saturated aqueous solutions, some of which are separated out, and bubbled with nitrogen for 20 minutes After removing air from the solution, 5 g of dopamine hydrochloride was added to the solution. Dissolve 4.7mL of methacrylic anhydride in 25mL of THF and add dropwise to the above solution. In order to keep the mixed solution properly alkaline, add 2M NaOH solution dropwise to adjust the pH of the solution to 8 or above, and stir the mixed solution for 14h under nitrogen atmosphere . The mixture was washed twice with 50 mL of ethyl acetate, the pH of the centrifuged aqueous solution was adjusted to below 2 with 6M HCl solution, and extracted three times with ethyl acetate. Then dry the obtained ethyl acetate layer solution with anhydrous magnesium sulfate, rotary evapora...
Embodiment example 2
[0026] Step 1: Dissolve 10g of sodium borate and 8g of sodium bicarbonate in 100mL of deionized water as the reaction medium (to protect the o-dihydroxyl group). Both sodium borate and sodium bicarbonate are saturated aqueous solutions, some of which are separated out, and bubbled with nitrogen for 20 minutes After removing air from the solution, 6.25 g of dopamine hydrochloride was added to the solution. Dissolve 4.7mL of methacrylic anhydride in 25mL of THF and add dropwise to the above solution. In order to keep the mixed solution properly alkaline, add 2M NaOH solution dropwise to adjust the pH of the solution to 8 or above, and stir the mixed solution for 14h under nitrogen atmosphere . The mixture was washed twice with 50 mL of ethyl acetate, the pH of the centrifuged aqueous solution was adjusted to below 2 with 6M HCl solution, and extracted three times with ethyl acetate. Then dry the obtained ethyl acetate layer solution with anhydrous magnesium sulfate, rotary evap...
Embodiment example 3
[0030]Step 1: Dissolve 10g of sodium borate and 8g of sodium bicarbonate in 100mL of deionized water as the reaction medium (to protect the o-dihydroxyl group). Both sodium borate and sodium bicarbonate are saturated aqueous solutions, some of which are separated out, and bubbled with nitrogen for 20 minutes After removing air from the solution, 5 g of dopamine hydrochloride was added to the solution. 4.7mL methacrylic anhydride was dissolved in 25mLTHF and added dropwise to the above solution. In order to keep the mixed solution properly alkaline, 2M NaOH solution was added dropwise to adjust the pH of the solution to 8 or above, and the mixed solution was stirred for 14h under nitrogen atmosphere. The mixture was washed twice with 50 mL of ethyl acetate, the pH of the centrifuged aqueous solution was adjusted to below 2 with 6M HCl solution, and extracted three times with ethyl acetate. Then dry the obtained ethyl acetate layer solution with anhydrous magnesium sulfate, rota...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com