A synthetic method of gold nanoparticle catalysts having a controllable dimension, gold catalysts and applications of the catalysts
A technology of gold nanoparticle and synthesis method, applied in the field of gold nanoparticle catalyst synthesis, can solve the problems of easy aggregation, poor stability, difficulty in large-scale production, poor monodispersity, etc., and achieves good high temperature stability, easy control, and avoidance of loss. and the effect of loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
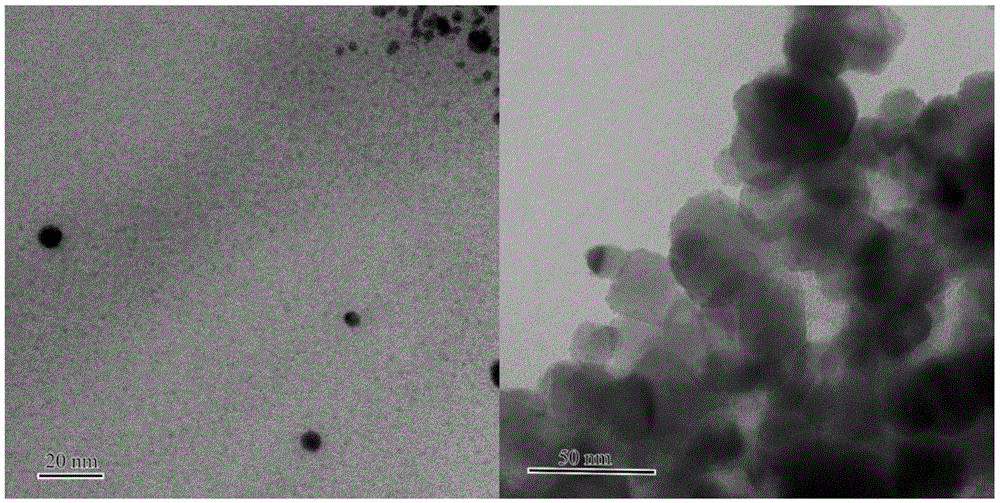
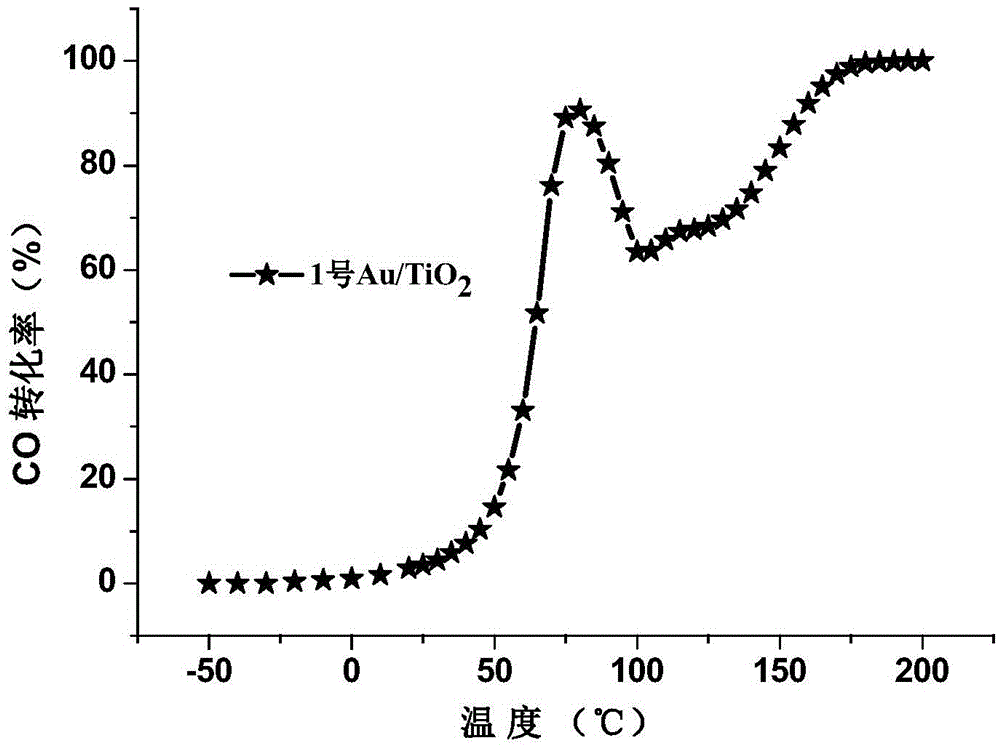
[0033] Embodiment 1: 1wt% Au / TiO with a diameter of about 1 nanometer of Au nanoparticles 2 Synthesis.
[0034] The mass concentration is 0.084mg.ml -1 The polyvinyl alcohol was added to a concentration of 1.6 mM HAuCl 4 solution, stirred for 30min to mix well. Weigh 10.7-11.0 mg of .075 times the molar equivalent of NaBH 4 Dissolve in 40mL of deionized water, take half of it and pour it into the above-mentioned protected chloroauric acid mixed solution for direct reduction to obtain a purple-brown colloidal solution. Add carrier TiO directly after reducing 3-5mim 2 , the measured pH value of the colloid is about 3, and the change is not significant after adding the carrier. Stirring and loading for 60 minutes, a blue-purple slurry was obtained, and after suction filtration, washing and drying in a vacuum oven, a blue-purple powder sample was obtained. Place the solid sample in a quartz tube at 3%% O 2 Oxidation treatment was carried out at 250°C for 4 hours at 250°C (a...
Embodiment 2
[0037] Embodiment 2: The proportion of reducing agent is different: 1wt% Au / TiO with a diameter of about 1.5 nanometers of Au nanoparticles 2 Synthesis.
[0038] The mass concentration is 0.084mg.ml -1 The polyvinyl alcohol was added to a concentration of 1.6 mM HAuCl 4 solution, stirred for 30min to mix well. Weigh 18.0-18.2 mg of 1.25 times the molar equivalent of NaBH 4 Dissolve in 40mL of deionized water, take half of it and pour it into the above-mentioned protected chloroauric acid mixed solution for direct reduction to obtain a purple-brown colloidal solution. Add carrier TiO directly after reducing 3-5mim 2 , the pH value of the colloid was measured to be around 3.2, and there was little change after adding the carrier. Stirring and loading for 60 minutes, a blue-purple slurry was obtained, and after suction filtration, washing and drying in a vacuum oven, a blue-purple powder sample was obtained. Place the solid sample in a quartz tube at 20% O 2 Oxidation trea...
Embodiment 3
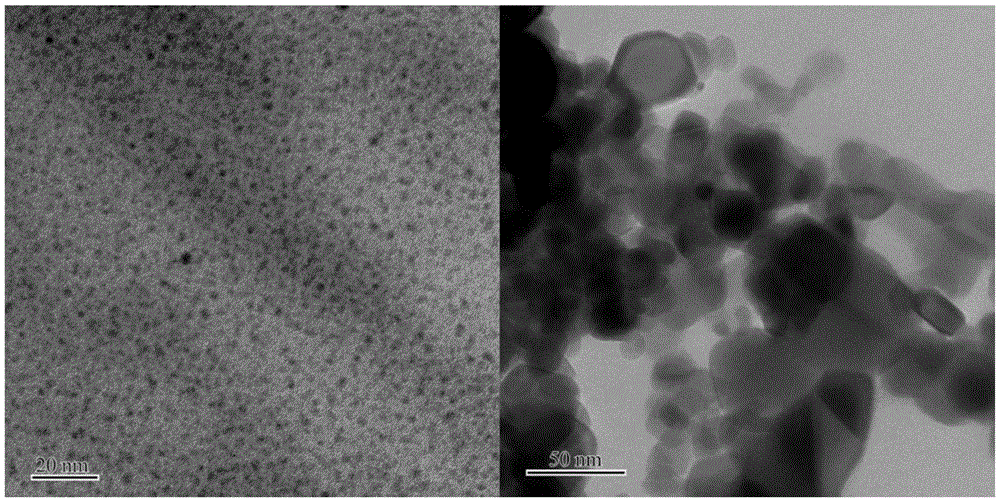
[0041] Embodiment 3: The proportion of reducing agent is different: 1wt% Au / TiO with Au nanometer particle diameter of about 3 nanometers 2 Synthesis.
[0042] The mass concentration is 0.084mg.ml -1 The polyvinyl alcohol was added to a concentration of 1.6 mM HAuCl 4solution, stirred for 30min to mix well. Weigh 36.2-36.4 mg of 5 times the molar equivalent of NaBH 4 Dissolve it in 20mL of deionized water, and pour it all at once into the above-mentioned protected chloroauric acid mixed solution for direct reduction to obtain a brown-red colloidal solution. Add carrier TiO directly after reducing 3-5mim 2 , the pH value of the colloid is measured at 5-6, and the change is not significant after adding the carrier. Stirring and loading for 60 minutes, a brown-red slurry was obtained, and after suction filtration, washing and drying in a vacuum oven, a brown-red powder sample was obtained. Place the solid sample in a quartz tube in 10% O 2 Oxidation treatment was carried o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com