Synthesizing method of phenoxyl radicals
A synthetic method and free radical technology, applied in the direction of organic chemical methods, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of rare synthesis reports of oxygen free radicals, and achieve high yield, simple synthesis process, and preparation process The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0016]
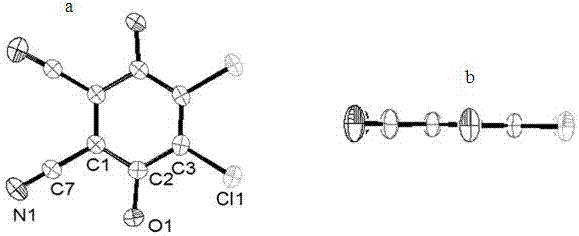
[0017] Add thienopyrrole (380mg, 2mmol) into a 250mL two-necked bottle, protect with inert gas, add 190mL of anhydrous and oxygen-free dichloromethane, keep away from light, and stir evenly. Control the temperature of the reaction system at -30~-40°C, stir for 30 minutes, and slowly add 190 μL Lewis acid BF 3 ·Et 2 O solution, reacted at low temperature for 3h, then naturally warmed up to room temperature and continued to react for 24h. DDQ (224mg, 1mmol) was dissolved in a small amount of dichloromethane and added to the reaction system, and continued to stir at room temperature for 24h. After the reaction was completed, the solvent was distilled off under reduced pressure, separated quickly by alumina column chromatography, and chloroform / methanol (4 / 1, v / v) was used as the eluent to collect the blue-purple components in the front section, which were recrystallized from methanol to obtain blue-gray Crystals, yield 68%.
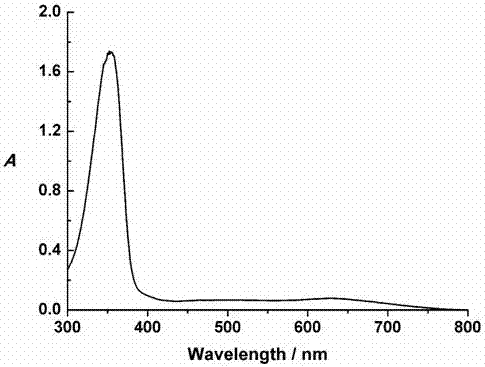
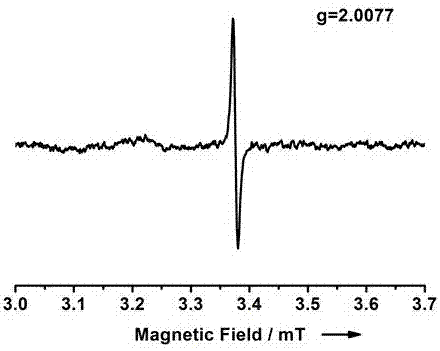
[0018] figure 1 It is the ultraviolet-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com