Method for preparing phosphatidic acid phospholipid compound by two-step method
A technology for polyenoic acid phospholipids and compounds is applied in the field of preparation of polyenoic acid phospholipids, can solve problems such as unfavorable large-scale production, and achieve the effects of low synthesis cost, simple preparation process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
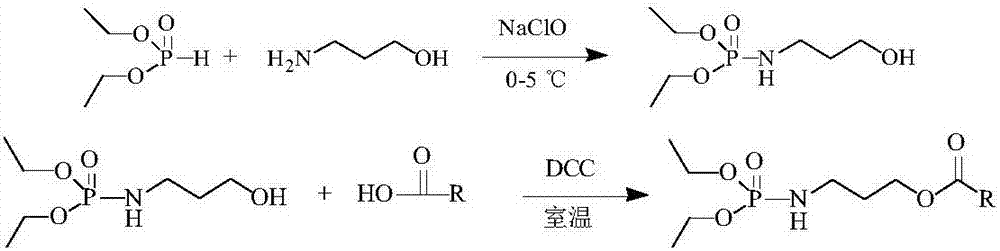
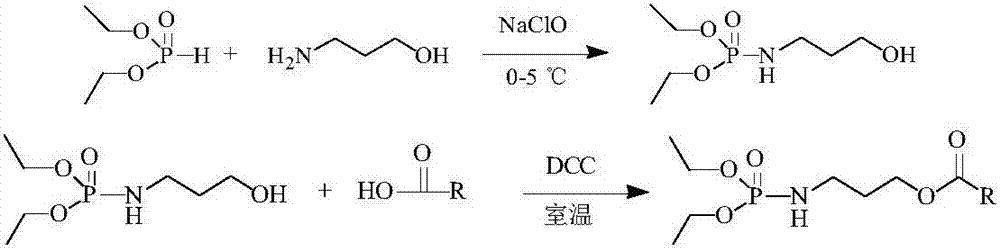
[0024] In a 200mL three-necked flask, add 0.1mol (7.5g) of 3-amino-1-propanol and 0.1mol (13.8g) of diethyl phosphite and place it in a low-temperature cooling pump at 0°C. While stirring, Slowly add 1.25mol (92.5g) sodium hypochlorite aqueous solution dropwise (the density is calculated as 1.0g / mL, and the NaClO content is calculated as 8%). After the reaction is completed, extract once with ethyl acetate, dry, and concentrate to obtain 8.6g (O, O) -Diethylphosphorylated-3-amino-1-propanol intermediate, ready to use. In another 100mL round bottom flask, add 0.03mol (10g) polyenoic acid (i.e. fatty acid mixture containing n-3 unsaturated hydrocarbon groups with 20 to 22 carbon atoms) and 0.03mol (6.33g) of the above-mentioned intermediate product, place At room temperature, under the condition of stirring, 0.036mol (7.42g) N,N-dicyclohexylcarbodiimide (DCC) solution dissolved in 30mL ethyl acetate was slowly added dropwise. After the reaction was completed, the solid N was rem...
Embodiment 2
[0026] In a 200mL three-necked flask, add 0.1mol (7.5g) 3-amino-1-propanol and 0.1mol (13.8g) diethyl phosphite and place it in a low-temperature cooling pump at 5°C, while stirring, Slowly add 1.0mol (74g) sodium hypochlorite aqueous solution dropwise (the density is calculated as 1.0g / mL, and the NaClO content is calculated as 8%). After the reaction is completed, extract once with ethyl acetate, dry, and concentrate to obtain 7.2g (O, O)- Diethylphosphorylated-3-amino-1-propanol intermediate, ready to use. In another 100mL round bottom flask, add 0.03mol (10g) polyenoic acid (i.e. fatty acid mixture containing n-3 unsaturated hydrocarbon groups with 20 to 22 carbon atoms) and 0.03mol (6.33g) of the above-mentioned intermediate product, place At room temperature, under the condition of stirring, slowly add 0.033mol (6.80g) DCC solution dissolved in 30mL ethyl acetate dropwise, after the reaction, remove the solid N,N-dicyclohexyl urea to obtain the target product polyenoic a...
Embodiment 3
[0028] In a 20L glass reactor bottle, add 10mol (750g) 3-amino-1-propanol and 10mol (1380g) diethyl phosphite, adjust the temperature of the low-temperature cooling circulation pump to make the temperature of the reaction solution in the kettle lower than 5°C, and Under the situation of stirring, slowly add dropwise 120mol (8.9kg) sodium hypochlorite aqueous solution (density is calculated by 1.0g / mL, and NaClO content is calculated by 8%), after the reaction finishes, ethyl acetate extracts once, is dried, concentrates and obtains 703g (O , O)-diethylphosphorylated-3-amino-1-propanol intermediate product for use. In another 5L Erlenmeyer flask, add 3mol (1kg) polyenoic acid (i.e. fatty acid mixture containing n-3 unsaturated hydrocarbon groups of 20 to 22 carbon atoms) and 3mol (633g) of the above-mentioned intermediate product, place at room temperature, Under the situation of stirring, slowly add dropwise the 4.2mol (865g) DCC solution that is dissolved in 1200mL ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com