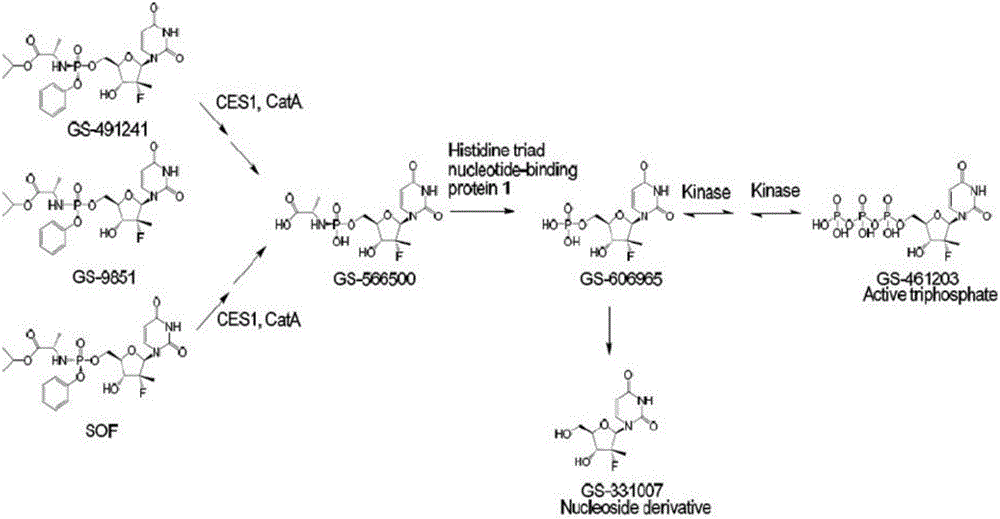
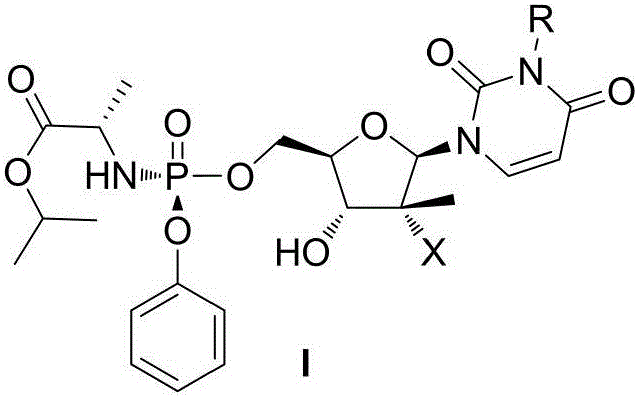
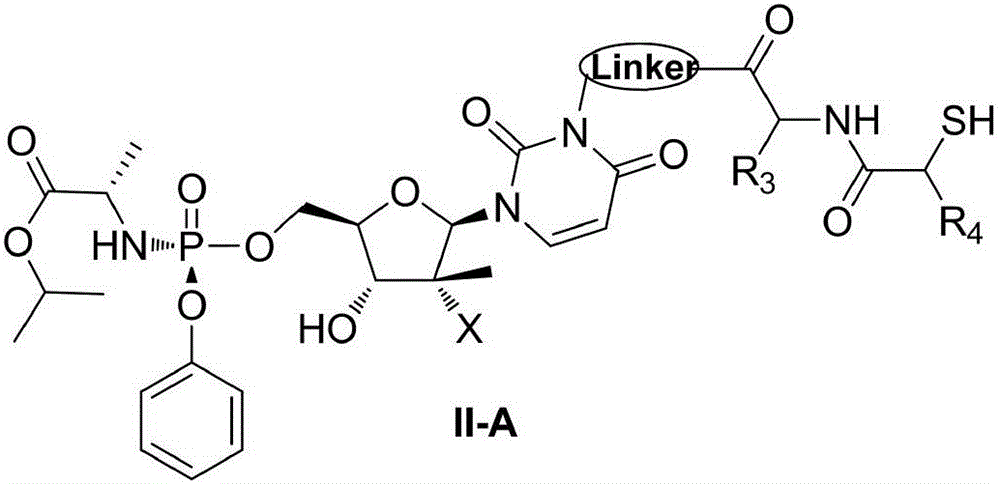
Prodrug, preparation method therefor, pharmaceutical compositions and use thereof
A prodrug and compound technology, applied in the field of anti-hepatitis C drugs, can solve the problems of increasing the economic burden of patients and poor patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0217] Embodiment 1: the synthesis of compound II-A-1
[0218] Method A:
[0219]
[0220] Step 1: Dissolve 529mg (1.0mmol) III-1 in 10mL of anhydrous acetone, add 680mg (1.5mmol) IV-A-1 and 414mg (3.0mmol) potassium carbonate at room temperature, heat, stir and reflux for 6h, and detect the reaction by TLC completely. After filtration, the filtrate was diluted with dichloromethane (30 mL), washed with water (10 mL) and saturated brine (10 mL) successively, the organic phase was separated, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography to obtain 710 mg of white solid V-A-1, with a yield of 75%, ESI-MS: m / z[M+H] + =947.
[0221] Step 2: Dissolve 200mg (0.21mmol) V-A-1 in 10mL of anhydrous dichloromethane, add 2mL at room temperature (volume ratio: dichloromethane / triisopropylsilane / trifluoroacetic acid=5 / 1 / 1) Solution, reacted for about 1 h, TLC det...
Embodiment 2
[0227] Embodiment 2: the synthesis of compound II-A-1a
[0228]
[0229] Compound VII-1 was freshly prepared according to step 1 of method B in Example 1 (3 mmol of compound III-1 as the starting material), dissolved in 12 mL of anhydrous dichloromethane (DCM), cooled in an ice bath under argon protection, Add 18 mg (0.15 mmol) 4-dimethylaminopyridine (DMAP) and 0.75 g (3.9 mmol) 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCHCl) successively, And 1.93g (3.6mmol) compound VIII-A-1a (prepared from chiral tiopronin, S configuration chiral purity 97%, analysis method: AD-H column temperature: 25C; detection wavelength: 210nm; flow rate: 1.0ml / min; mobile phase: n-Hex:EtOH:TFA=90:10:0.1 isocratic elution), then slowly rise to room temperature and stir the reaction for about 12 hours, and the reaction is complete as detected by TLC. Add 30 mL of dichloromethane, wash with saturated aqueous sodium bicarbonate solution and saturated brine successively, separate t...
Embodiment 3
[0231] Embodiment 3: the synthesis of compound II-A-1b
[0232]
[0233] According to Step 1 and Step 2 of Example 2, VIII-A-1b (prepared from chiral tiopronin, R configuration chiral purity 95%, analysis method: AD-H column temperature: 25C; detection wavelength: 210nm ; Flow rate: 1.0ml / min; Mobile phase: n-Hex:EtOH:TFA=90:10:0.1 isocratic elution) replace VIII-A-1a, can prepare 8.7 with 5.3g (10mmol) compound III-1 g Intermediate IX-A-1b, yield 73% (two steps).
[0234] According to step 3 of Example 2, the compound IX-A-1b (2.9 mmol) continued to remove the protecting group, and 1.7 g of white solid product II-A-1b could be prepared, with a yield of 86%. ESI-MS: m / z[ M+H] + =705.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com