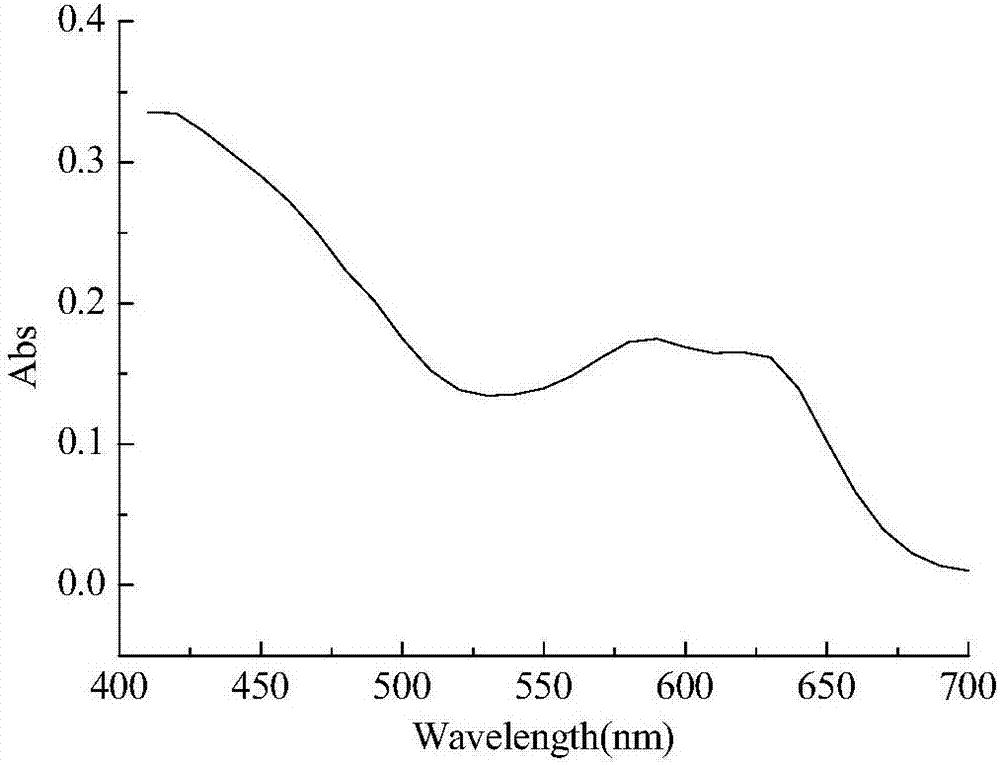
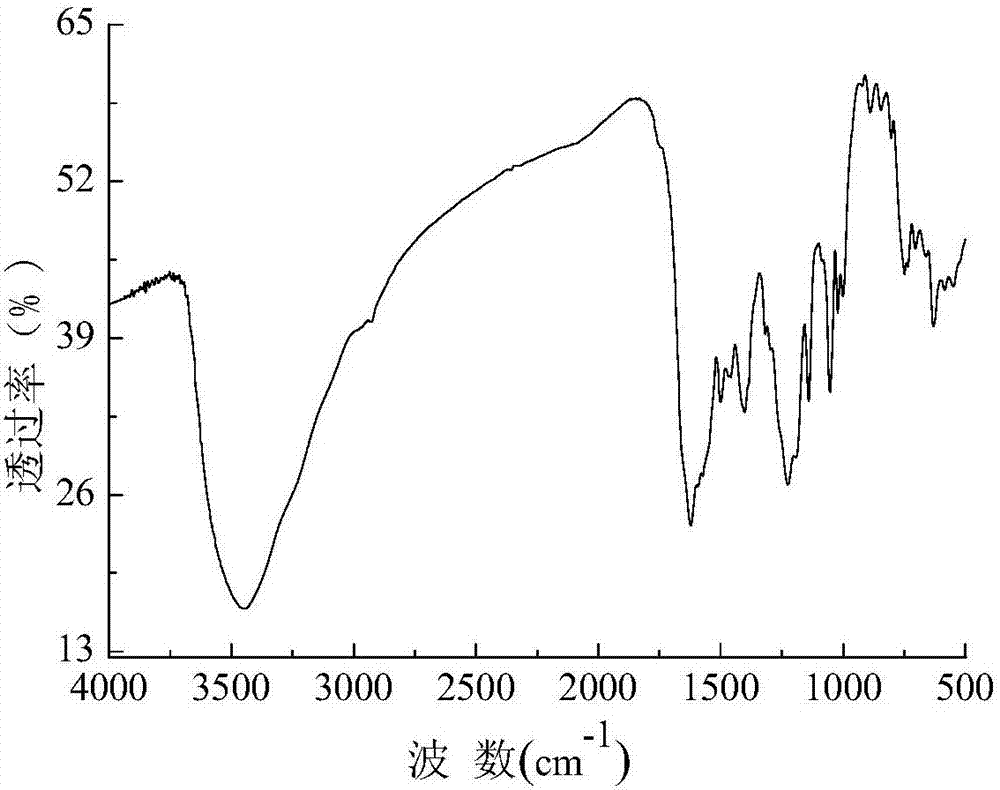
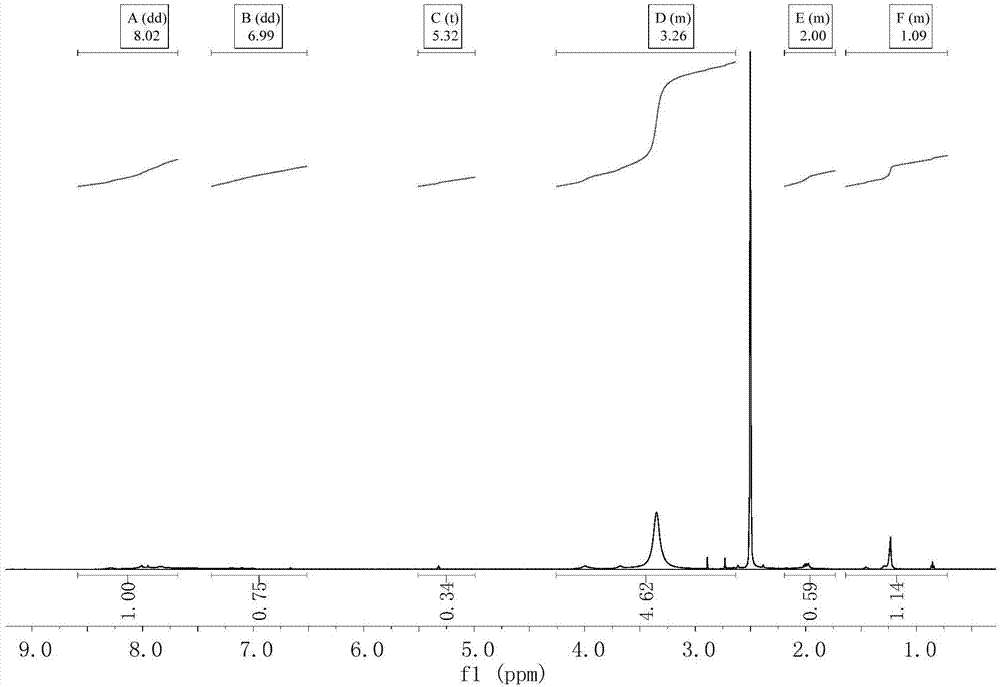
Bisazo multi-chromophoric group greenish black active dye, preparation method and application thereof
A reactive dye and disazo technology, applied in reactive dyes, azo dyes, dyeing methods, etc., can solve the problems of low color fixation rate of reactive dyes, low color fixation rate of dyes, large amount of sewage, etc., and achieve dyeing balance time short, simple preparation process, and convenient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment prepares the dye structure (D-1) of para-ester coupling, and the structural formula is as follows:
[0041]
[0042] (1) Preparation of a condensate: 18.4 parts of cyanuric chloride are uniformly dispersed in 50 parts of ice-water mixture in a beaker. Weigh 53.15×0.99 parts of 100% 3,.5-diamino-2,4,6-trimethylbenzenesulfonic acid amino-substituted anthraquinone derivative intermediate, add 100 parts of water to it, stir evenly, place Cool to 5°C in an ice bath. Add this 3,.5-diamino-2,4,6-trimethylbenzenesulfonic acid amino-substituted anthraquinone derivative intermediate solution dropwise into cyanuric chloride in an ice-water bath, at pH=4.5, React for 3 hours at T=0°C, and detect the end point of the reaction with thin-layer chromatography (developing agent: n-propanol: isobutanol: ethyl acetate: water = 2:4:1:3; the above is the volume ratio), the reaction equation As follows.
[0043]
[0044] (2) Preparation of the dicondensate: Weigh 21×...
Embodiment 2
[0055] Using 3-vinylsulfone sulfate aniline (meta-position ester) to carry out acidic coupling, the obtained dye structure (D-2) (yield rate 78.4%) is as follows:
[0056]
[0057] The synthetic method is the same as in Example 1, and the raw material para-ester is replaced with a meta-ester, and all the other reaction conditions are the same as in Example 1.
[0058] When the dye is dyed at 60°C to dye pure cotton fabrics, bright and bright dark green is also obtained, and the color fastness is excellent, as shown in Table 2.
[0059] Table 2 Color fastness properties of dyed fabrics
[0060]
Embodiment 3
[0062] Using p-ester for acidic coupling and using 2,4-diaminobenzenesulfonic acid for dicondensation, the obtained dye structure (D-3) (yield 83.6%) is as follows:
[0063]
[0064] The synthesis method is the same as in Example 1, except that 3,5-diaminobenzoic acid is replaced with 2,4-diaminobenzenesulfonic acid, and the rest of the reaction conditions are the same as in Example 1.
[0065] When dyeing pure cotton fabric at 60°C, bright and bright dark green is also obtained, and the color fastness is excellent, as shown in Table 3.
[0066] Table 3 Color fastness properties of dyed fabrics
[0067]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com