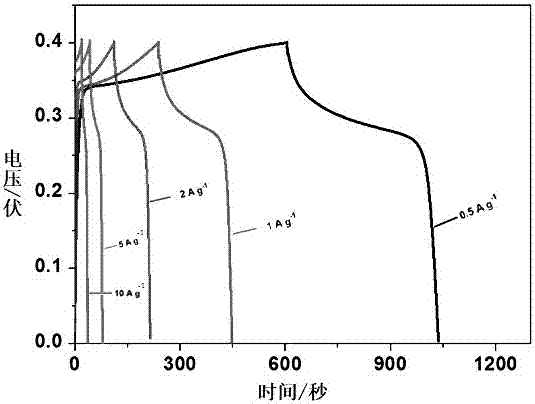
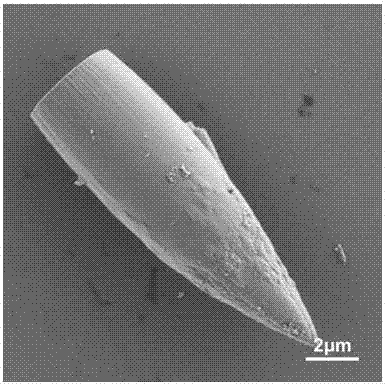
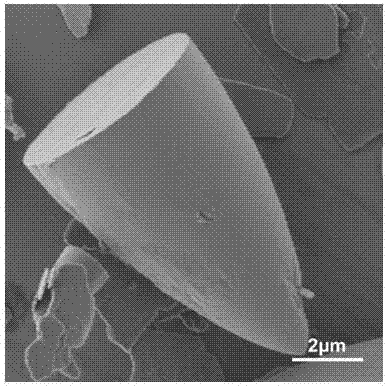
Preparation method of bullet-shaped cobaltous phosphate ammonium nitrate particles with multilayer scales and application thereof
A cobalt nickel ammonium phosphate and bullet head technology, applied in the field of materials and chemical synthesis, can solve the problems of particle size, poor control of phase, affecting electrochemical energy storage performance, harsh synthesis conditions of composite metal phosphate materials, etc. Easy operation, uniform size, good distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Weigh 0.3 g of ammonium phosphate, 0.24 g of nickel nitrate hexahydrate and 0.06 g of cobalt nitrate hexahydrate, and pour them all into 10 mL of deionized water and 10 mL of ethylene glycol, and stir the mixture evenly at room temperature to make Mix well and fully react.
[0033] The mixing molar ratio of nickel nitrate and cobalt nitrate in the above materials is 4:1.
[0034] (2) Put the mixed system after the reaction in the previous step into an ultrasonic machine for ultrasonication, so that the components are evenly dispersed.
[0035] (3) Then place the uniformly dispersed system at 200° C. for hydrothermal reaction for 45 hours.
[0036] (4) After the hydrothermal reaction is completed, the reaction solution is naturally cooled to room temperature, the reactant is taken out, and the precipitate is obtained after standing for stratification, washed with water and ethanol, and then dried to obtain blue-gray cobalt nickel ammonium phosphate powder.
Embodiment 2
[0038] (1) Weigh 0.3 g of ammonium phosphate, 0.24 g of nickel nitrate hexahydrate and 0.06 g of cobalt nitrate hexahydrate, and pour them all into 10 mL of deionized water and 10 mL of ethylene glycol, and stir the mixture evenly at room temperature to make Mix well and fully react.
[0039] (2) Put the mixed system after the reaction in the previous step into an ultrasonic machine for ultrasonication, so that the components are evenly dispersed.
[0040] (3) Then place the uniformly dispersed system at 200° C. for hydrothermal reaction for 20 hours.
[0041] (4) After the hydrothermal reaction is completed, the reaction solution is naturally cooled to room temperature, the reactant is taken out, and the precipitate is obtained after standing for stratification, washed with water and ethanol, and then dried to obtain blue-gray cobalt nickel ammonium phosphate powder.
Embodiment 3
[0043] (1) Weigh 0.3 g of ammonium phosphate, 0.3 g of nickel nitrate hexahydrate and 0.04 g of cobalt nitrate hexahydrate, and pour them all into 10 mL of deionized water and 10 mL of ethylene glycol, and stir the mixture evenly at room temperature. Let it mix well and fully react.
[0044] The mixing molar ratio of nickel nitrate and cobalt nitrate in the above materials is 7.5:1.
[0045] (2) Put the mixed system after the reaction in the previous step into an ultrasonic machine for ultrasonication, so that the components are evenly dispersed.
[0046] (3) Subsequently, the uniformly dispersed system was placed at 200° C. for hydrothermal reaction for 10 hours.
[0047] (4) After the hydrothermal reaction is completed, the reaction solution is naturally cooled to room temperature, the reactant is taken out, and the precipitate is obtained after standing for stratification, washed with water and ethanol, and then dried to obtain blue-gray cobalt nickel ammonium phosphate po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific capacitance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com