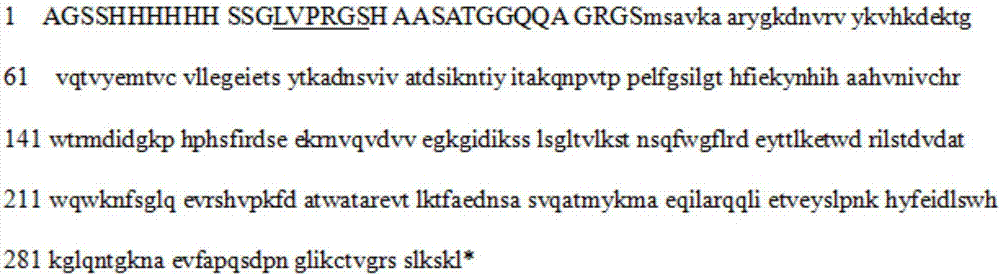Preparation and application of uric acid lowering oral medicine
A technology for reducing uric acid and uricase, which is applied in the field of preparation of oral uric acid-lowering drugs, can solve the problems of insufficient support and failure to explain the source of uricase, and achieve high safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation and activity determination of uricase.
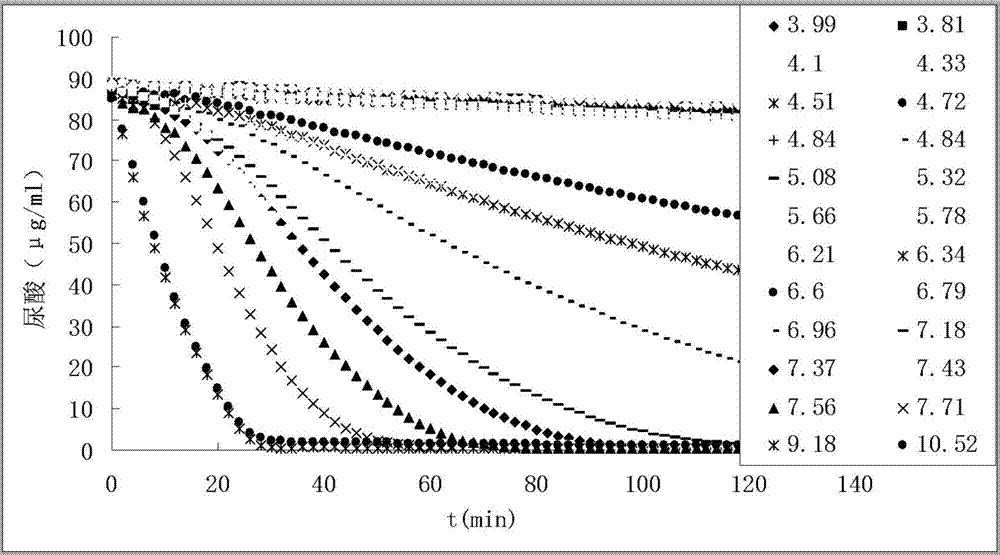
[0042] According to the sequence (XM_002377830.1) of the mRNA of Aspergillus flavus uricase, the full length of the coding sequence was chemically synthesized, and then connected to the pET28a vector (with Karabacteria sp. protein resistance), Uricase-pET28a was obtained, and the correct encoding of uricase protein (see figure 1 ) vector to transfect Escherichia coli DH5a to obtain a large number of vectors, the DH5a bacteria were cultured in large quantities and the bacteria were recovered, and the Uricase-pET28a vector was extracted after the bacteria were lysed. The Uricase-pET28a vector was purified and introduced into BL21(DE3) Escherichia coli, and positive bacteria were obtained by karimycin resistance screening. Then use the LB medium containing karimycin resistance (50 μg / ml) to cultivate Uricase-pET28a-BL21 (DE3) bacteria in large quantities on a shaker (160 rpm) at 37 ° C. When the bacteria propa...
Embodiment 2
[0044] Example 2 Preparation of Bacillus subtilis macromolecular extract with uricase activity.
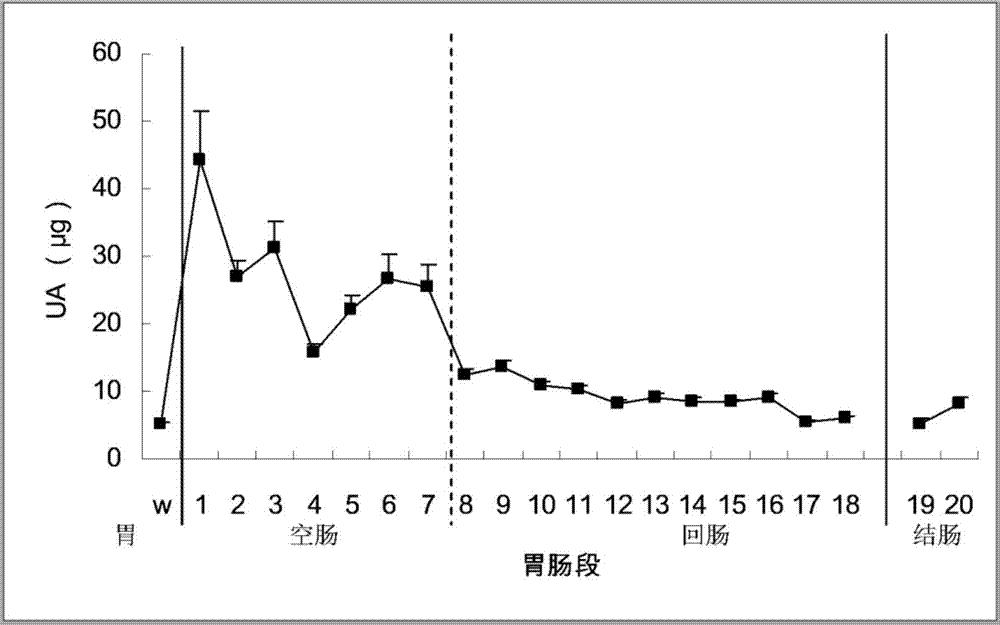
[0045] Inoculate live medical or edible Bacillus subtilis into 1L sterilized medium (containing 3.0g beef extract, 5.0g peptone per liter, pH 6.8±0.2), shake at 37°C (160rpm) until the turbidity OD600 reaches 2 Above, then centrifuge for 10 minutes to recover the bacteria (5000rpm, 4°C), and the macromolecular extraction sample is called the pure Bacillus subtilis group from the bacteria. Another bottle of bacteria was cultured in parallel, the method was the same as before, but 10 g of uric acid was added during the culture, and the bacteria were also recovered. The macromolecular extraction sample came from the uric acid-induced Bacillus subtilis group of the bacteria. The recovered bacteria were resuspended with pH 7.40 phosphate buffer, crushed with an ultrasonic crusher, and centrifuged to obtain supernatant (to remove particles such as cell nuclei). Put the supernatant in a...
Embodiment 3
[0050] Example 3 Preparation of yeast macromolecule extract with uricase activity.
[0051] Inoculate edible beer yeast live bacteria (yeast powder) into 1L sterilized medium (each liter of culture medium contains 3.0g of yeast extract, 3.0g of malt extract, 10.0g of glucose, 5.0g of peptone, pH6.2±0.2) , Shake at 37°C (160rpm) until the turbidity OD600 reaches above 2, then centrifuge for 10 minutes to recover the yeast (5000rpm, 4°C), the macromolecule extraction sample is called the pure yeast group. Another bottle of yeast was cultured in parallel, the method was the same as before, but 10 g of uric acid was added during the cultivation, and the yeast was also recovered, and the macromolecule extraction sample came from the uric acid-induced yeast group of the bacteria. The recovered bacteria were resuspended with pH 7.40 phosphate buffer, crushed with a high-pressure crusher, and then centrifuged to obtain supernatant (to remove particles such as cell nuclei). Put the su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com