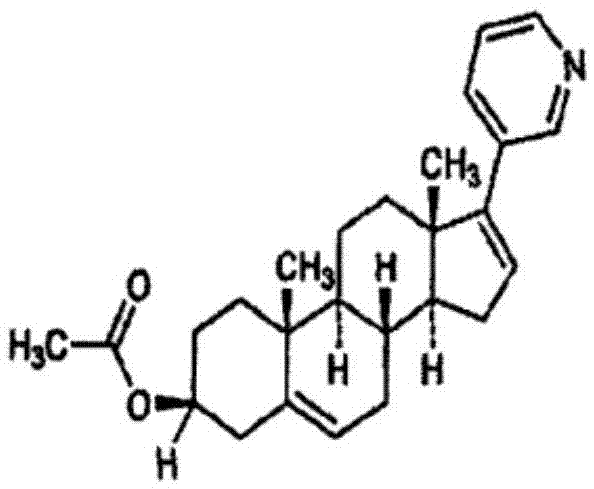
Abiraterone acetate sublingual tablet and preparation method thereof
A technology of abiraterone acetate and sublingual tablet, applied in the field of medicine, can solve problems such as unfavorable workshop safety production, worker labor protection, limited dissolution improvement, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Preparation Process:
[0028] Dissolve abiraterone acetate, hydroxypropyl cellulose, and povidone in methanol, add lactose, stir evenly, fill in blisters, and then freeze-dry in a freeze dryer. The cover is press-sealed and packaged.
Embodiment 2
[0030]
[0031] Preparation Process:
[0032] Prescription quantity Weigh abiraterone acetate, hydroxypropyl cellulose, povidone and dissolve in dichloromethane, add mannitol, stir evenly, fill in blisters, then place in a lyophilizer to lyophilize, freeze After drying, the blister is sealed and packaged.
Embodiment 3
[0034]
[0035]
[0036] Preparation Process:
[0037] Dissolve abiraterone acetate, hydroxypropyl cellulose, and povidone in chloroform, add lactose, stir evenly, fill in blisters, and then freeze-dry in a freeze dryer. The cover is press-sealed and packaged.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com