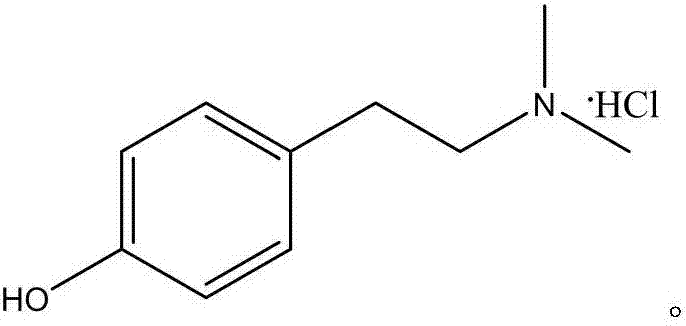
Synthetic method of hordenine hydrochloride
A technology of hordenine hydrochloride and a synthesis method, which is applied in the field of synthesis of hordenine hydrochloride, can solve the problems of unfavorable industrial production, complex process, high production cost, etc., and achieves convenience for industrial production, simple synthesis process, and high production cost. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] Amination reaction
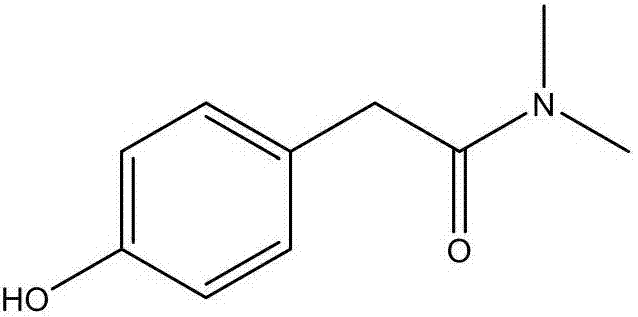
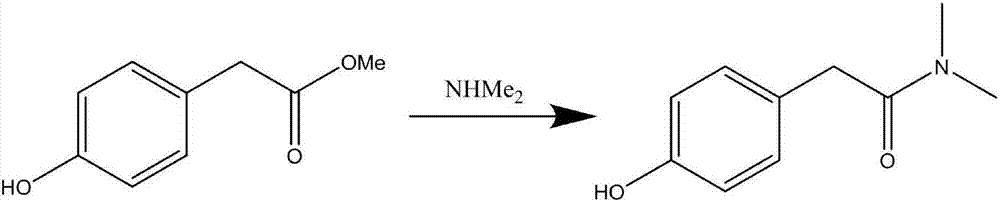
[0032] Add 166 g (1 mol, 1 equivalent) of methyl p-hydroxyphenylacetate and 500 ml of methanol successively into the reaction flask, pass through 115 g (2.5 mol, 2.5 equivalents) of dimethylamine, react at 50-60 ° C for 10 hours, and then evaporate the methanol to dryness. Then add 500ml of water to stir for crystallization, cool down to 20-25°C, filter, and dry to obtain 148g of intermediate N,N-dimethyl-p-hydroxyphenylacetamide, with a yield of 82.7%.
example 2
[0034] Amination reaction
[0035] Add 166 g (1 mol, 1 equivalent) of methyl p-hydroxyphenylacetate and 500 ml of methanol successively into the reaction flask, pass through 280 g (6 mol, 6 equivalents) of dimethylamine, react at 50-60°C for 10 hours, evaporate the methanol to dryness, and then Add 500ml of water to stir for crystallization, cool down to 20-25°C, filter, and dry to obtain 146g of intermediate N,N-dimethyl-p-hydroxyphenylacetamide, with a yield of 81.5%.
example 3
[0037] Amination reaction
[0038] Add 166 g (1 mol, 1 equivalent) of methyl p-hydroxyphenylacetate and 500 ml of methanol successively into the reaction flask, pass through 450 g (10 mol, 10 equivalents) of dimethylamine, react at 15-25 ° C for 20 hours, evaporate the methanol to dryness, and then Add 500ml of water to stir for crystallization, cool down to 20-25°C, filter, and dry to obtain 144g of intermediate N,N-dimethyl-p-hydroxyphenylacetamide, with a yield of 80.4%.
[0039] The chemical reaction equation of above-mentioned embodiment 1-3 is:
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com