Method for synthesizing terbutaline and bovine serum albumin conjugate
A technology for bovine serum albumin and terbutaline, which is applied in the field of synthesizing terbutaline and bovine serum albumin conjugates, can solve problems such as affecting health and poisoning, achieves short time, simple synthesis process, and simplifies synthesis effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve BSA (66.43 mg, 1 μmol) in PBS (pH=6.0) buffer, then add terbutaline (8.23 mg, 30 μmol), stir to dissolve, then add dropwise 40% formaldehyde solution (30 μl), at room temperature Stir the reaction for 24 hours to obtain the reaction solution; transfer the reaction solution to a dialysis bag, put the dialysis bag into a beaker filled with distilled water, and dialyze for 3 days under stirring at room temperature; centrifuge the dialysis product in a high-speed refrigerated centrifuge, and collect The clear liquid was lyophilized in vacuum for 12 hours to obtain a white powder of the conjugate (26.3 mg, yield: 39.42%).
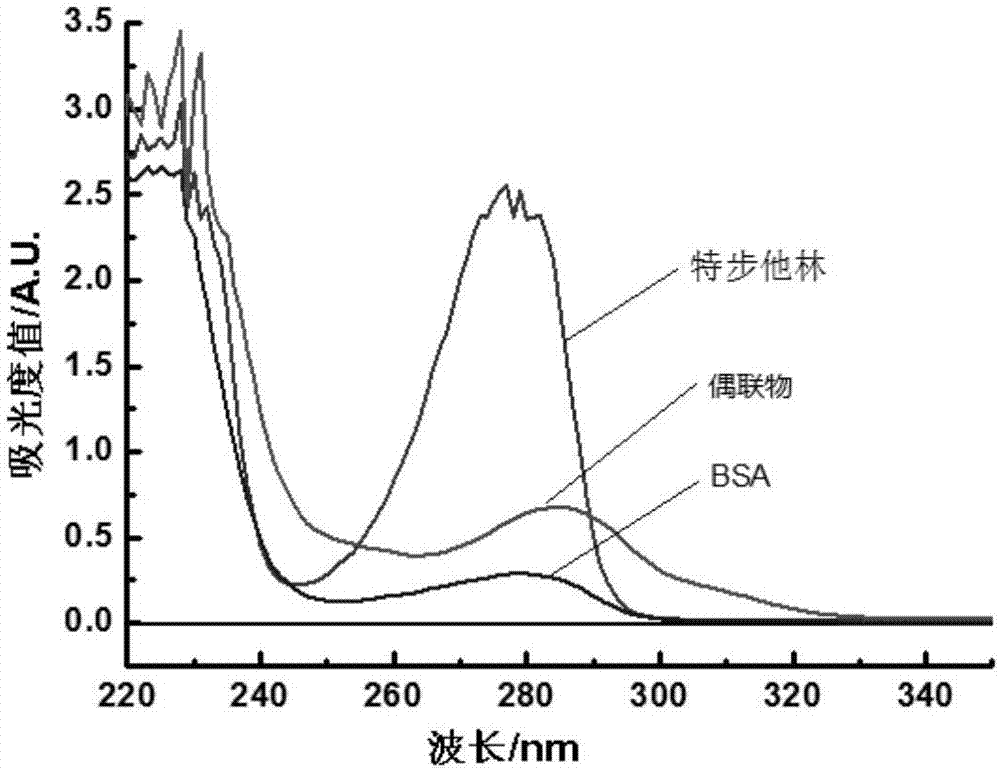
[0022] BSA, terbutaline and the conjugate were prepared with PBS (pH=6.0) respectively to prepare 0.5 mg / ml solutions for later use. First use PBS buffer solution (pH=6.0) to balance the baseline in the wavelength range of 220-400nm, then scan the prepared 3 solutions respectively, and record the absorbance values scanned at each wavelength ban...
Embodiment 2
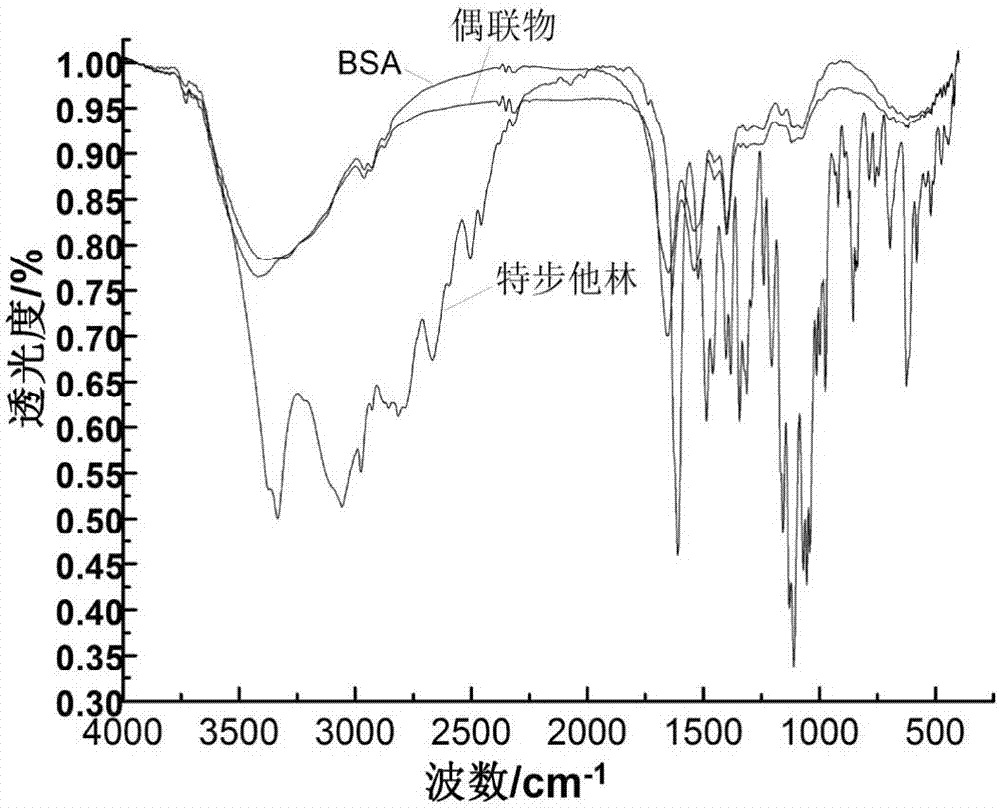
[0024] After compressing BSA, terbutaline and the conjugated product with KBr, the infrared spectrum was scanned. Although the infrared absorption curve of the conjugated product is very similar to that of BSA, some absorption peaks have moved or The enhancement was presumed to be due to the coupling of BSA to terbutaline. From the infrared spectrum of terbutaline, it can be seen that the absorption of 1,3,5-trisubstituted aromatic hydrocarbons: 695cm -1 The strong absorption peak of the 1,3,5-trisubstituted compound at , the skeleton vibration of the benzene ring is at 1610cm -1 、1522cm -1 and 1486cm -1 These three absorption peaks can also be seen in -C(CH 3 ) 3 The methyl group of the structure has two absorption peaks with different intensities, namely about 1382cm -1 、1344cm -1 , 3334cm in the picture -1 The broad peak is the stretching vibration of the hydroxyl group, and the infrared spectra of the conjugates have similar absorption peaks. In addition, in the in...
Embodiment 3
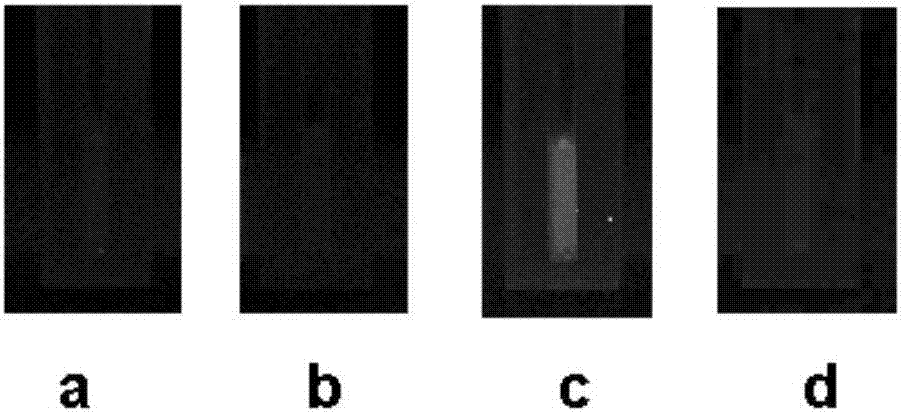
[0026] image 3 Shown are the fluorescence effects of BSA, terbutaline, conjugates, and equimolar mixed solutions of terbutaline and BSA under 365nm ultraviolet light. The substances in the solutions of the first three groups are all 0.1 mM. The sum of BSA and terbutaline in the mixed solution was 0.1 mM. It can be clearly seen that only the conjugate has fluorescence, and it can also be inferred that the conjugation of terbutaline and BSA is successful. See image 3 , in the figure: (a) the fluorescence effect diagram of BSA, (b) the fluorescence effect diagram of terbutaline, (c) the fluorescence effect diagram of the conjugate, (d) the fluorescence effect of the mixture of terbutaline and BSA picture.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com