Aromatic hydrocarbon receptor and immune detection method of antibody mediated therewith
An immunodetection method and an aromatic hydrocarbon receptor technology, which can be used in biological testing, material inspection products, etc., and can solve the problems of environmental persistence, inability to degrade easily, and toxic reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
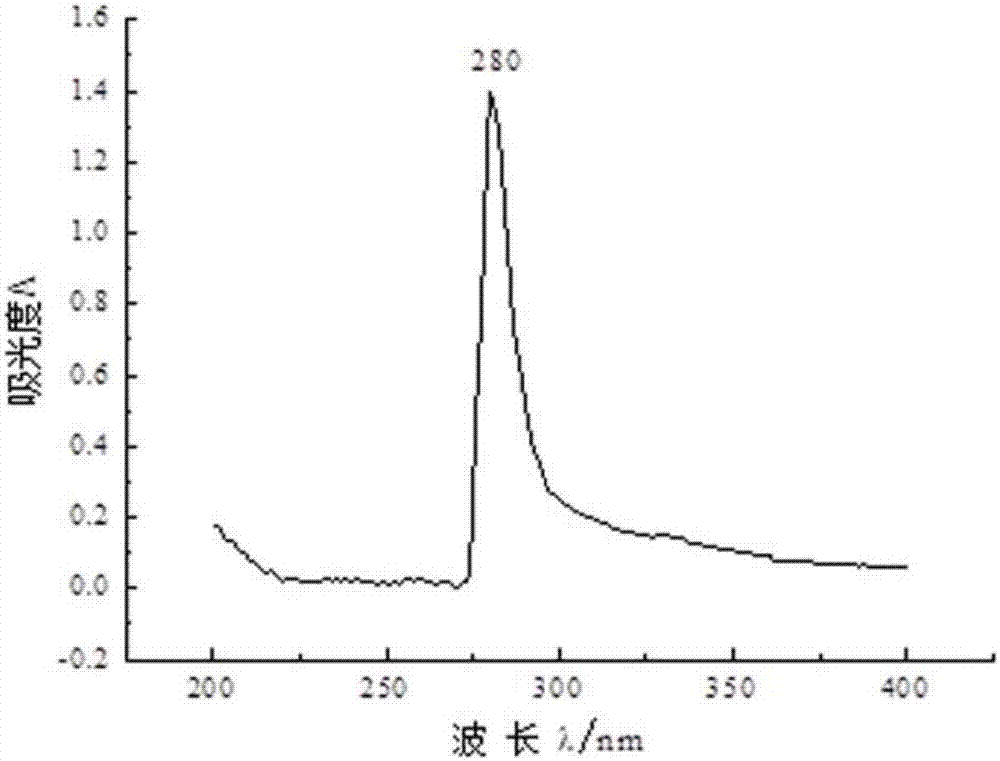
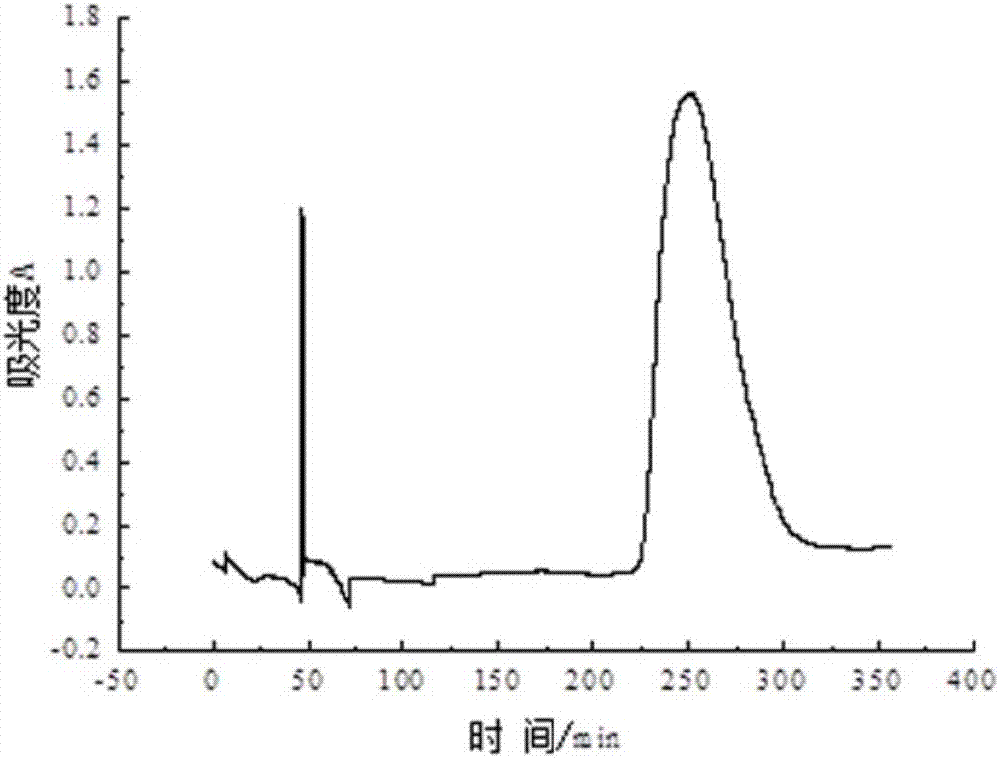
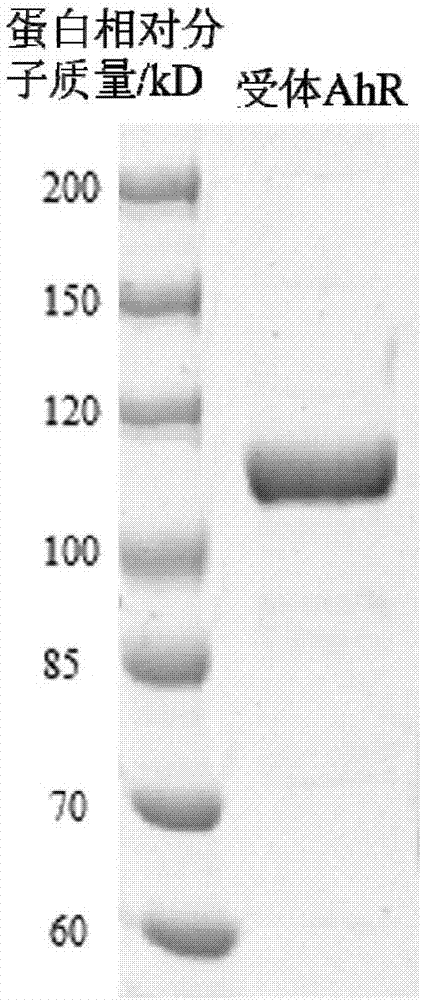
[0031] 1. Isolation and purification of aryl hydrocarbon receptor (AhR) in carp
[0032] 1. Extraction of AhR-Containing Cytosol
[0033] Take the carp liver domesticated in the water containing Ant, first wash the blood on the surface of the liver with normal saline, then dry the surface water, weigh, and then add HEDG buffer at a mass-volume ratio of 1g:4ml for grinding. Liver tissue homogenate was obtained (all operations were performed on ice). The homogenate was centrifuged at 13000 rmp at 4°C for 1 h, and the supernatant was aspirated, which was the cytosol containing the receptor. It can be stored at -80°C and can be used for further experiments.
[0034] 2. Separation and purification of AhR
[0035] The protein was purified using ion exchange chromatography. The sample was equilibrated with the starting buffer overnight. The sample was carefully added to the surface of the resin along the tube wall with a dropper, and the lower nozzle valve was opened. When the sa...
Embodiment 2
[0039] 1. Drawing of standard curve and detection limit of indirect competitive ELISA method
[0040] Add 100 μL of coated antigen diluted 2000 times to each well of the 96-well ELISA plate, and coat at 4°C for 12 hours overnight. Pour out the liquid in the well, shake and wash with washing solution PBS-T for 3 min, repeat 3 times, pat dry for use; add 200 μL of blocking liquid gel to each well, seal at 37 °C for 60 min, pour out the liquid in the well, repeat the above washing steps, Pat dry until use; dilute primary antibody to 0.275 μg mL -1 , add 50 μL to each well, and dilute Ant with 10% volume of acetone solution to prepare a series of concentrations of 10, 20, 50, 100, 200, 500, 1000, 10000ng / mL, add 50 μL to each well, and set a blank control at the same time. Incubate at °C for 60 min. Repeat the above washing steps, pat dry for use; dilute the enzyme-labeled secondary antibody to 0.1 μg·mL -1 , add 100 μL to each well, incubate at 37°C for 60 min, repeat the abov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com