Method of preparing ruthenium nitrosyl nitrate
A technology of ruthenium nitrosyl nitrate and sodium nitrite, which is applied in the field of directly preparing ruthenium nitrosyl nitrate with metal ruthenium powder, can solve the problems of high acidity, hindrance to application, high price, etc., and achieve simplified synthesis steps, shortened reaction time, The effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
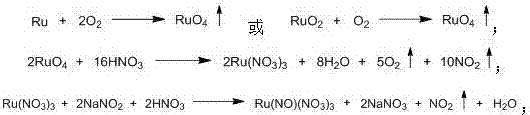
[0017] Weigh 5.0 g of ruthenium powder and 1.23 g of vanadium pentoxide and place them in a porcelain crucible, then put them in a microwave oven; feed air and keep the air velocity at 4000m 3 h -1 , calcined under 1000W microwave power for 15min, and then calcined under 1400W microwave power for 100min to oxidize metal ruthenium powder; the produced RuO 4 The gas is sequentially introduced into three absorption bottles containing 27.7g of nitric acid solution with a mass concentration of 45%, and the temperature of the nitric acid solution is controlled at 70°C to obtain Ru(NO 3 ) 3 acid solution.
[0018] The above obtained Ru(NO 3 ) 3 The acid solution was placed in a double-neck round bottom flask, and 5.12g of NaNO was slowly added 2 powder, stirred and heated at 80°C and condensed to reflux for 6 hours, the color of the solution gradually changed to deep red black, cooled the solution, and placed it in a separatory funnel, added 83mL of anhydrous ether for extractio...
Embodiment 2
[0020] Weigh 10.0 g of ruthenium powder and 2.4 g of vanadium pentoxide and place them in a porcelain crucible, then put them into a microwave oven, and feed air to keep the air velocity at 4500m 3 h -1 , calcined at 1000W microwave power for 17min, and then calcined at 1500W microwave power for 80min to oxidize metal ruthenium powder. will produce RuO 4 The gas is sequentially introduced into three absorption bottles containing 56.0 g of nitric acid solution with a mass concentration of 50%, and the temperature of the nitric acid solution is controlled at 70°C to obtain Ru(NO 3 ) 3 acid solution.
[0021] The above obtained Ru(NO 3 ) 3 The acid solution is all placed in a double-neck round bottom flask, slowly add 14.7g of KNO 2 powder, stirred and heated to reflux at 80°C for 7 hours, the color of the solution gradually turned dark red and black, cooled the solution, and placed it in a separatory funnel, added 168 mL of anhydrous ether for extraction 4 times, collected...
Embodiment 3
[0023] Weigh 15.0 g of ruthenium powder and 3.86 g of vanadium pentoxide and place them in a porcelain crucible, then put them in a microwave oven, and feed air to keep the air flow rate at 5000 m 3 h -1 , calcined under 1000W microwave power for 19min, and then calcined under 1600W microwave power for 60min to oxidize metal ruthenium powder. will produce RuO 4 The gas is sequentially introduced into three absorption bottles containing 85.1 g of nitric acid solution with a mass concentration of 55%, and the temperature of the nitric acid solution is controlled at 70°C to obtain Ru(NO 3 )3 acid solution.
[0024] The above obtained Ru(NO 3 ) 3 The acid solution was placed in a double-necked round bottom flask, and 20.5g of NaNO was slowly added 2 powder, stirred and heated to reflux at 80°C for 8 hours, the color of the solution gradually turned dark red and black, cooled the solution, and placed it in a separatory funnel, added 255 mL of anhydrous ether for extraction 3 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com