Polymer with main chain containing sulphone unit as well as preparation method and application thereof
A technology of polymers and compounds, which is applied in the fields of electrical components, semiconductor/solid-state device manufacturing, circuits, etc., can solve problems such as low triplet energy level, difficulty in achieving delayed fluorescence emission, and device performance not reaching the expected level. Efficiency roll-off, simple preparation method, and high external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
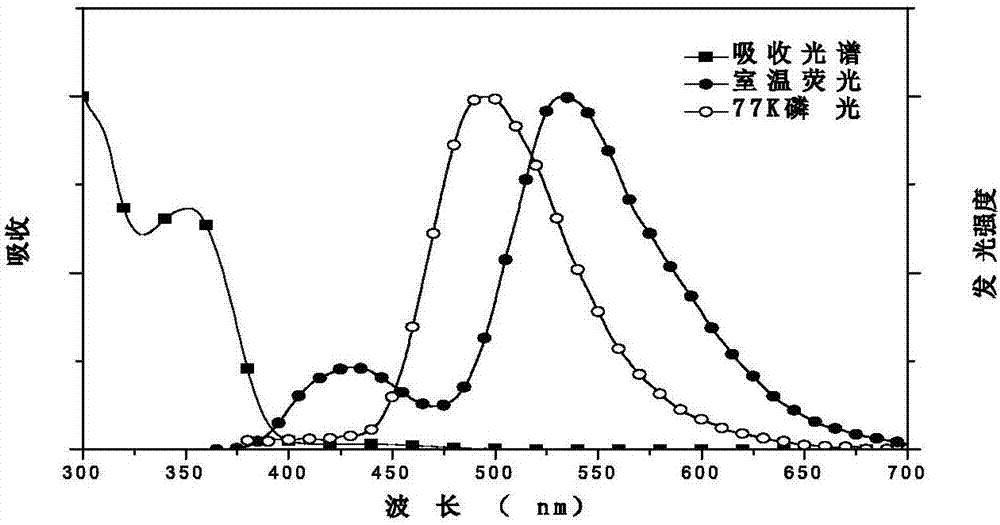
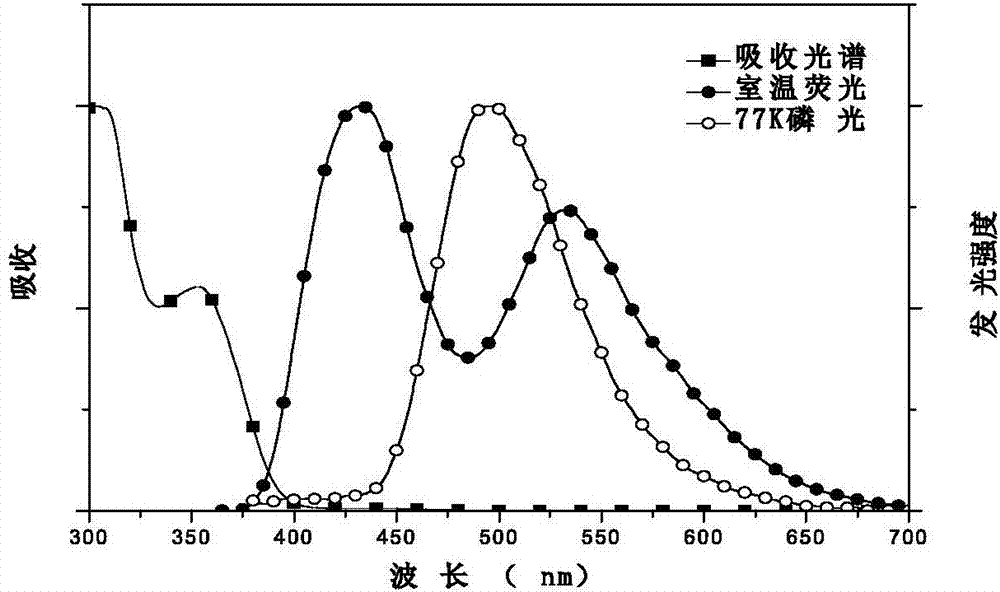
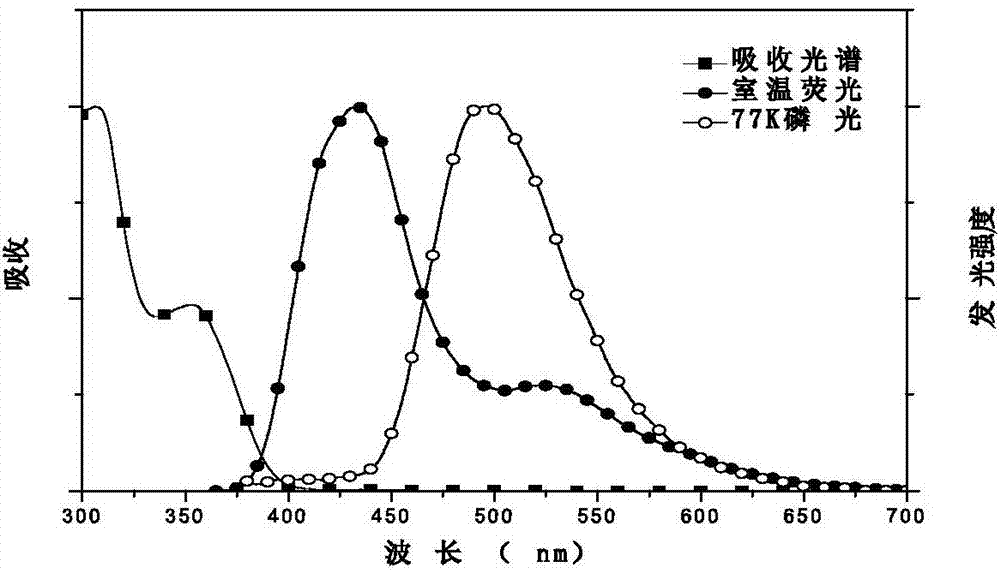
Image
Examples
preparation example Construction
[0077] The present invention also provides a method for preparing the polymer containing sulfone units in the main chain of the present invention, comprising:
[0078] Copolymerize the compound with the structure of formula (II), the compound with the structure of formula (III) and the compound with the structure of formula (IV) to obtain the polymer with the structure shown in formula (I);
[0079]
[0080] Among them, R 1 , R 2 , R 3 C1-C30 alkyl, C1-C30 alkoxy or C6-C35 substituted aryl;
[0081] A is a C6-C50 aryl group or a C3-C45 heteroaryl group containing an electron-withdrawing group;
[0082] x is 0.001
[0083] n is 1-200;
[0084] According to the present invention, the present invention will have the compound of formula (II) structure, the compound of formula (III) structure and the compound of formula (IV) structure to copolymerize, obtain the polymer of structure shown in formula (I); Wherein, in the structure R 1 , R 2 , R 3 , the selection r...
Embodiment 1
[0089] Example 1: Synthesis of Polymer PCSAPTP25
[0090] The preparation process is shown in the following formula:
[0091]
[0092] The specific steps are:
[0093] Bis(4-(3-bromo-6-(2-ethyl)hexylcarbazole)phenyl)sulfone (139.6mg, 0.15mmol), 2,7-dibromo-9,9-dihexyl-10-( 4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-9,10-dihydroacridine (122.2mg, 0.15mmol), 9-(4-( Dodecyloxy)phenyl)-3,6-dipinacol borate-9-hydrocarbazole (203.9 mg, 0.3 mmol) and bis(tri-o-methylphenylphosphine)palladium dichloride ( 2.4mg, 0.003mmol) was added to a 100mL Schlenk bottle, the gas was changed three times, and argon protection was added, and oxygen-depleted tetrahydrofuran (10mL) and oxygen-depleted potassium phosphate aqueous solution (2mL, 2M) were added, and the reaction was refluxed at 80°C for 12h; Phenylboronic acid (69mg, 0.6mmol) dissolved in 1mL of tetrahydrofuran was added to the reaction solution, reacted for 6h, then 1mL of bromobenzene was added to the reaction solution, and react...
Embodiment 2
[0095] Example 2: Synthesis of Polymer PCSAPTP10
[0096] The preparation process is shown in the following formula:
[0097]
[0098] The specific steps are:
[0099] Bis(4-(3-bromo-6-(2-ethyl)hexylcarbazole)phenyl)sulfone (223.4mg, 0.24mmol), 2,7-dibromo-9,9-dihexyl-10-( 4-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-9,10-dihydroacridine (49.0mg, 0.06mmol), 9-(4-( Dodecyloxy)phenyl)-3,6-dipinacol borate-9-hydro-carbazole (203.9 mg, 0.3 mmol) and bis(tri-o-methylphenylphosphine)palladium dichloride (2.4mg, 0.003mmol) was added to a 100mL Schlenk bottle, and the gas was exchanged 3 times, protected by argon, and oxygen-depleted tetrahydrofuran (10mL) and oxygen-depleted potassium phosphate aqueous solution (2mL, 2M) were added, and the reaction was refluxed at 80°C for 12h; Phenylboronic acid (69 mg, 0.6 mmol) dissolved in 1 mL of tetrahydrofuran was added to the reaction solution, and reacted for 6 hours, then 1 mL of bromobenzene was added to the reaction solution, and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com