Synthesis method for 3-(trifluoroacetyl)indole derivative
A technology of indole derivatives and synthesis methods, which is applied in the field of synthesizing 3-indole derivatives by carbon-hydrogen bond functionalization reaction, can solve the problems that have not been reported in the literature, and achieve high yield, economical raw materials, and easy operation. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
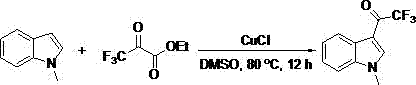
[0015] Example 1: Synthesis of 3-(trifluoroacetyl)-1-methylindole
[0016]
[0017] Add dimethyl sulfoxide (2 mL) to a 25 mL pressure-resistant reaction tube, N - Methylindole (39.3 mg, 0.3 mmol), ethyl trifluoropyruvate (102.0 mg, 0.6 mmol), cuprous chloride (44.78 mg, 1.5 eq.), magnetically stirred in an oil bath at 80 °C for 12 Hour. After the reaction was completed, most of the solvent was evaporated under reduced pressure, and the remaining mixed solution was separated and purified by column chromatography with petroleum ether / ethyl acetate (10:1) as the eluent to obtain the desired product, which was light yellow Solid, 57.9 mg, yield 85%.
[0018] Its NMR data are as follows:
[0019] 1 H NMR (300 MHz, CDCl 3 ) δ= 8.04−8.02 (m, 1H), 7.30−7.27 (m, 3H), 3.68(s, 3H), 2.73 (s, 3H); 13 C NMR (CDCl 3 , 75 MHz) δ= 175.0 (q, J CF = 35.6 Hz), 150.2, 136.7, 125.0, 123.1, 121.5, 120.6 (q, J CF = 4.3 Hz), 117.2 (q, J CF =288.0 Hz), 109.7, 107.6, 29.7, 12.9.
Embodiment 2
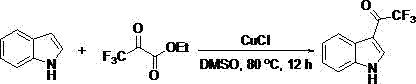
[0020] Example 2: Synthesis of 3-(trifluoroacetyl)indole
[0021]
[0022] Add dimethyl sulfoxide (2 mL), indole (35.1 mg, 0.3 mmol), ethyl trifluoropyruvate (102.0 mg, 0.6 mmol), cuprous chloride (44.78 mg, 1.5 eq.), and reacted for 12 hours in an 80°C oil bath with magnetic stirring. After the reaction was completed, most of the solvent was evaporated under reduced pressure, and the remaining mixed solution was separated and purified by column chromatography with petroleum ether / ethyl acetate (10:1) as the eluent to obtain the desired product, which was light yellow Solid, 35.8 mg, yield 56%.
[0023] Its NMR data are as follows:
[0024] 1 H NMR (300 MHz, CDCl 3 ) δ =12.75 (s, 1 H), 8.49 (s, 1 H), 8.20 (d, J =5.6 Hz, 1 H), 7.60 (d, J = 6.4 Hz, 1 H), 7.36−7.30 (m , 2 H); 13 C NMR (DMSO-d 6 ,75 MHz) δ 174.0 (q, J CF = 33.6 Hz), 137.6 (q, J CF = 4.8 Hz), 136.7, 125.8,124.4, 123.5, 121.2, 117.0 (q, J CF = 289.8 Hz), 113.1, 108.9.
Embodiment 3
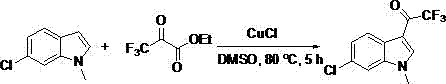
[0025] Example 3: Synthesis of 3-(trifluoroacetyl)-1-methyl-6-chloroindole
[0026]
[0027] Add dimethyl sulfoxide (2 mL) to a 25 mL pressure-resistant reaction tube, N -Methyl-6-chloroindole (49.5 mg, 0.3 mmol), ethyl trifluoropyruvate (102.0 mg, 0.6 mmol), cuprous chloride (44.78 mg, 1.5 eq.), in an oil bath at 80 °C The reaction was stirred magnetically for 12 hours. After the reaction was completed, most of the solvent was evaporated under reduced pressure, and the remaining mixed solution was separated and purified by column chromatography with petroleum ether / ethyl acetate (10:1) as the eluent to obtain the desired product, which was light yellow Solid, 55.6 mg, yield 71%.
[0028] Its NMR data are as follows:
[0029] 1 H NMR (300 MHz, CDCl 3 ) δ = 8.30 (d, J = 8.8 Hz, 1 H), 7.89 (s, 1 H), 7.39 (d, J = 1.6 Hz, 1 H), 7.34 (dd, J = 2.0 Hz, 2.0 Hz, 1 H), 3.88 (s, 3 H); 13 C NMR (CDCl 3 , 75 MHz) δ= 174.7 (q, J CF = 34.9 Hz), 138.7 (q, J CF = 4.8 Hz), 137.8,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com