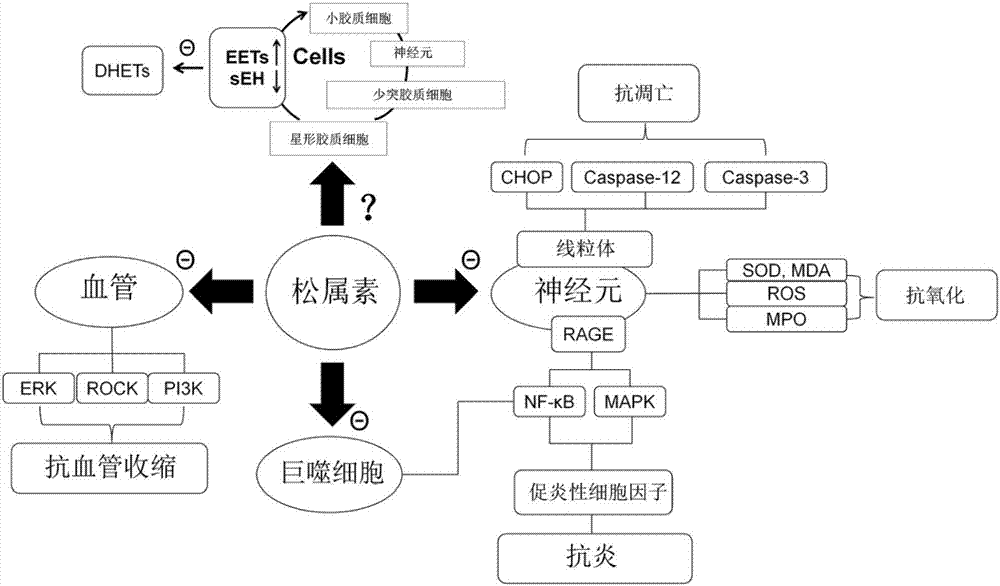
Application of pinocembrin to preparation of medicines for treating demyelination diseases
A technology for demyelinating diseases and therapeutic drugs, applied in the field of pharmaceutical preparations and pinine salts containing pinine, pinine precursor or pinine hydrate, can solve unclear problems and achieve relief from medical treatment cost, promotion of myelin sheath regeneration, effect of mature purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
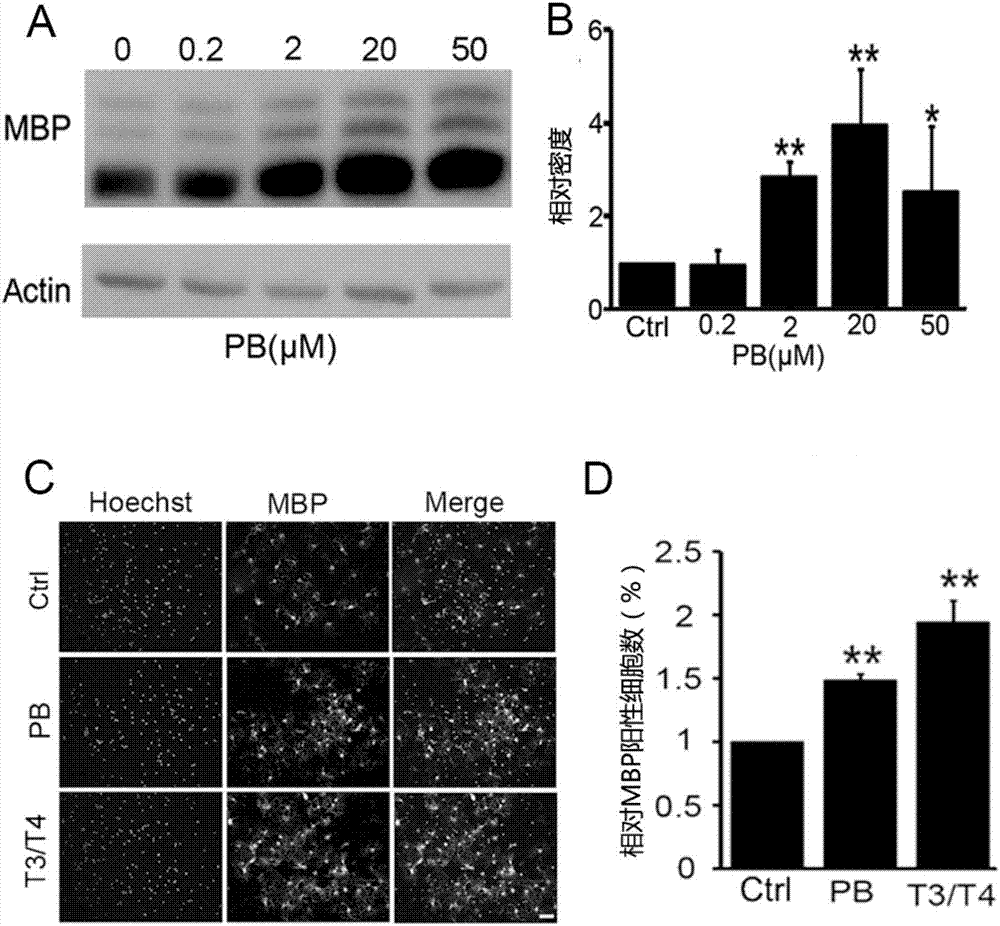
[0030] Example 1: The promotion effect of Pinustin (PB) on the differentiation of oligodendrocyte precursor cells (OPCs)
[0031] 1. Experimental method:
[0032] In order to study and confirm the effect of pininetin on the differentiation of OPCs, qRT-PCR, Western blot, and immunocytochemistry were used to confirm that pininetin can promote the differentiation and maturation of OPCs from the mRNA level, protein level and morphological level.
[0033] 1. Experimental cells: primary cultured oligodendrocyte precursor cells (OPCs).
[0034]2. Reagents and consumables commonly used in the experiment: DMEM culture medium and Neurobasal / B27 culture medium were purchased from Gibco Company in the United States; fetal bovine serum FBS was purchased from PAA Company in Australia; 0.25% Trypsin trypsin, L-polylysine (poly- L-lysine, PLL) was purchased from Sigma, USA; Trizol Reagent was purchased from Invitrogen. Other routine reagent materials in the laboratory were purchased from S...
Embodiment 2
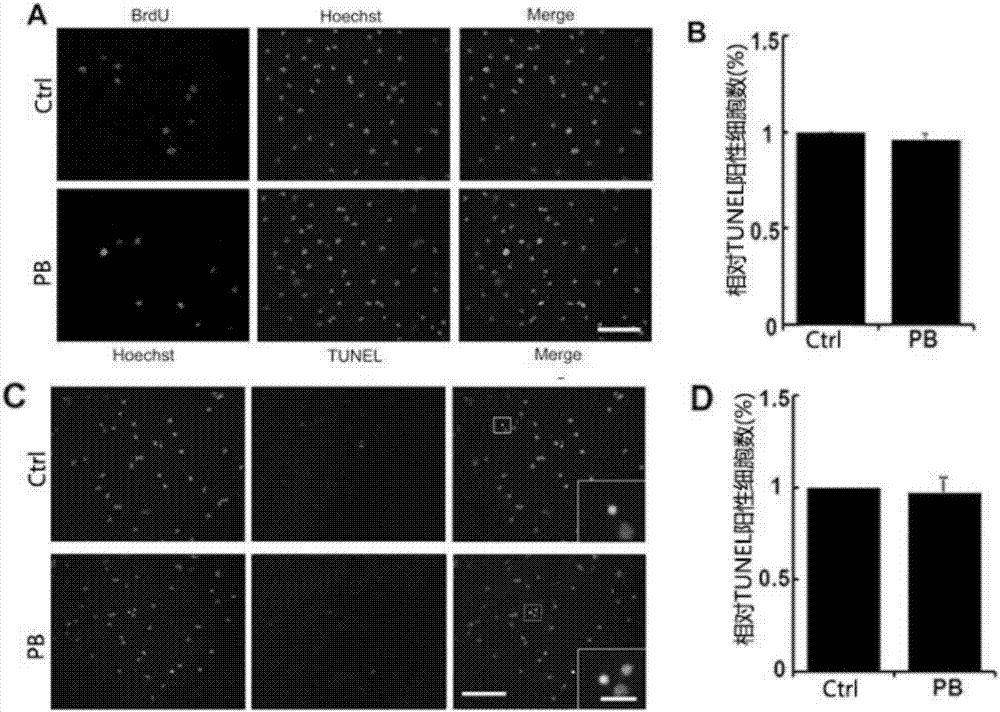
[0053] Example 2: Pinustin does not affect cell proliferation and survival while promoting OPCs differentiation
[0054] 1. Experimental method:
[0055] In order to verify whether pinoquinone will affect other changes in cells while promoting OPCs differentiation, BrdU binding assay and TUNEL assay were used to study cell proliferation and apoptosis, reflecting the influence of drugs on cells from the two indicators.
[0056] 1. Experimental cells: primary cultured oligodendrocyte precursor cells (OPCs).
[0057] 2. Experimental reagents, consumables and instruments: DMEM culture medium, Neurobasal / B27 were purchased from Gibco, USA; fetal bovine serum FBS was purchased from PAA, Australia; 0.25% Trypsin, L-polylysine (poly-L -lysine, PLL) was purchased from Sigma, USA; Trizol Reagent was purchased from Invitrogen. Other routine reagent materials in the laboratory were purchased from Shanghai Sinopharm Group, and cell culture dishes and plates were purchased from Corning In...
Embodiment 3
[0069] Example 3: The promoting effect of pinoquinone on the functional restoration of EAE
[0070] 1. Experimental method:
[0071] Based on the results of in vitro experiments, in order to further verify the pharmacological effects of pinoquinone on the inflammation-induced demyelination model, we successfully induced the EAE animal model and administered it therapeutically. On the 10th day of MOG-induced EAE model, observed Dosing was started after the onset of symptoms in the mice, and the administration continued until the 25th day to evaluate whether the drug could treat / relieve the disease.
[0072] 1. Experimental animals: C57BL / 6J adult female mice were purchased from Shanghai Slack Company and Nanjing University Model Animal Center. All animal experiments were performed in accordance with the animal management regulations and ethical requirements of the Animal Management Committee of the Second Military Medical University of the Chinese People's Liberation Army.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com