A kind of preparation method of pentafluorophenol
A technology for pentafluorophenol and pentafluorobenzene boric acid is applied in the field of preparation of pentafluorophenol, can solve the problems of difficult water removal, high recovery cost, troublesome recovery and the like, and achieves the effects of stable process, convenient operation and reduction of operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
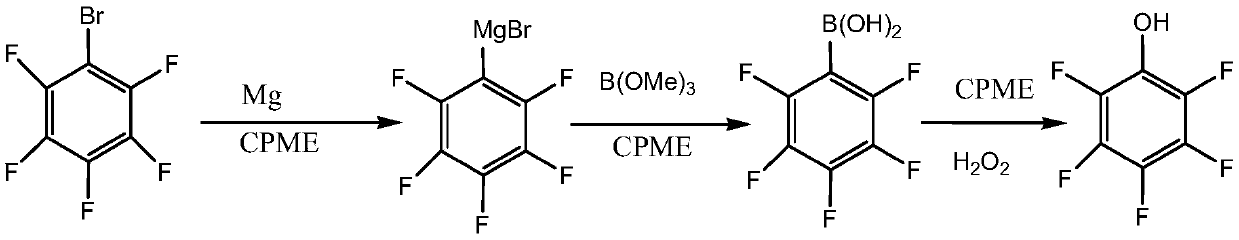
[0034] Step (1): Dry the 1000ml four-necked bottle, replace it with nitrogen, start stirring to maintain good nitrogen protection, add 21.6g (0.891mol) of magnesium chips, 468g of cyclopentyl methyl ether (CPME), 0.02 g iodine, heat up to 40-50°C, add 10g (0.04mol) pentafluorobromobenzene, stir the reaction, after the reaction is triggered, add the remaining 190g (0.77mol) of pentafluorobromobenzene dropwise, after the addition, the temperature rises at 40-50 Incubate at ℃ for 2 hours to obtain Grignard reagent.
[0035] Step (2): Add 133g of cyclopentyl methyl ether and 97g (0.94mol) of trimethyl borate into a 2000ml four-neck flask, cool down to -10-0°C, and drop the Grignard reagent prepared in the above step (1) Add in, then heat to room temperature and keep it warm for 2 hours, then acidify with 10% hydrochloric acid to pH<1, separate layers, and the oil layer is directly used in the next step.
[0036] Step (3): Add the oil layer obtained in step (2) into a 1000ml four-...
Embodiment 2
[0039] Step (1): Dry the 1000ml four-necked bottle, replace it with nitrogen, start stirring to maintain a good nitrogen protection, add 21.6g (0.891mol) of magnesium chips in the four-necked bottle, and recover the cyclopentyl methyl ether (Example 1 The cyclopentyl methyl ether recovered by the method, the water content is 85ppm) 468g, 0.02g iodine, the temperature is raised to 40-50°C, 10g (0.04mol) pentafluorobromobenzene is added, and the reaction is stirred. After the reaction is triggered, the remaining 190 g (0.77 mol) of bromopentafluorobenzene was added, and after the addition was completed, it was incubated at 40-50° C. for 2 hours to obtain the Grignard reagent.
[0040] Step (2): add the cyclopentyl methyl ether that 103g reclaims in the 2000ml four-necked bottle (the cyclopentyl methyl ether that the method described in embodiment 1 reclaims, water content 85ppm) and fresh cyclopentyl methyl ether 30g and 97g (0.94mol) trimethyl borate, lower the temperature to -...
Embodiment 3
[0044] Step (1): Dry the 1000ml four-necked bottle, replace it with nitrogen, start stirring to maintain good nitrogen protection, add 19.7g (0.81mol) of magnesium chips, 468g of cyclopentyl methyl ether (CPME), 0.02 g iodine, heat up to 25-35°C, add 10g (0.04mol) pentafluorobromobenzene, stir the reaction, after the reaction is triggered, add the remaining 190g (0.77mol) of pentafluorobromobenzene dropwise, after the addition is completed, the temperature rises at 25-35 Incubate at ℃ for 2 hours to obtain Grignard reagent.
[0045] Step (2): Add 133g of cyclopentyl methyl ether (CPME) and 83.6g (0.81mol) of trimethyl borate into a 2000ml four-necked bottle, cool down to 0-10°C, and put the lattice prepared in the above step (1) Add the Nitrile reagent dropwise, then heat to room temperature and keep it warm for 2 hours, then acidify with 10% hydrochloric acid to pH<1, separate the layers, and the oil layer is directly used in the next step.
[0046] Step (3): Add the oil lay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com