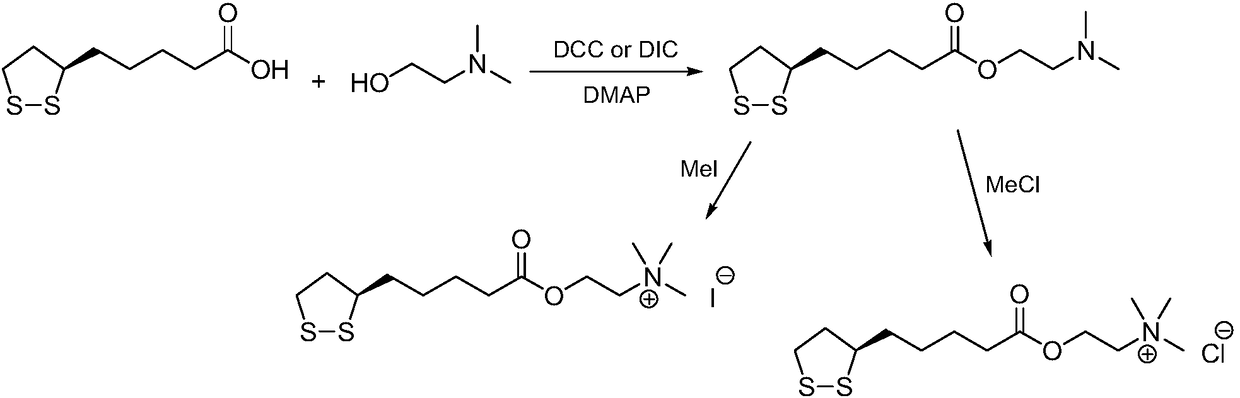
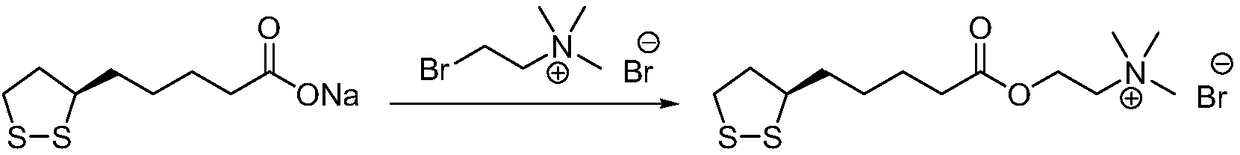
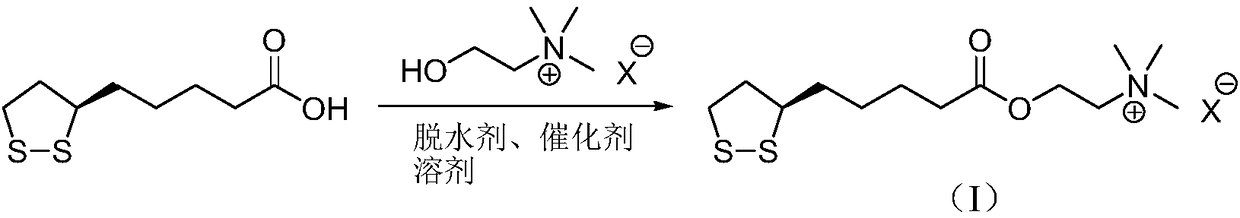
The method for preparing r-lipoic acid choline ester halide
A lipoic acid choline and halide technology, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of unspecified preparation conditions and operations, and achieve the effects of safe use, easy-to-obtain reagents, and reasonable technical solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Method A): Add R-lipoic acid (10.0g, 48.5mmol) and toluene (200mL) into a reaction flask, stir to dissolve, add N,N'-dicyclohexylcarbodiimide (12.0g, 58.2mmol) and 4-Dimethylaminopyridine (1.2g, 9.7mmol) was cooled in an ice bath, choline chloride (15.2g, 109.1mmol) was added, and the reaction mixture was reacted at 35°C for 18h. Post-treatment and purification, the crude product was recrystallized with isopropanol to obtain R-lipoic acid choline ester chloride, a light yellow solid (14.8g), with a yield of 93%, and the reaction formula was:
[0028]
Embodiment 2
[0030] Method B): Add R-lipoyl chloride (10.0g, 44.5mmol) and chloroform (200mL) into a reaction flask, stir to dissolve, add N,N-diethylaniline (26.6g, 178.0mmol), cool in an ice bath, Choline chloride (18.6g, 133.5mmol) was added, and the reaction mixture was reacted at 20°C for 12h. Post-treatment and purification, the crude product was recrystallized with isopropanol to obtain R-lipoic acid choline ester chloride, a light yellow solid (12.7g), with a yield of 87%, and the reaction formula was:
[0031]
Embodiment 3
[0033] Method A): Add R-lipoic acid (10.0g, 48.5mmol) and 1,2-dichloroethane (200mL) into the reaction flask, stir to dissolve, add N,N'-carbonyldiimidazole (8.6g, 53.3mmol ) and 2,6-lutidine (0.5g, 4.8mmol), cooled in an ice bath, added choline bromide (26.8g, 145.4mmol), and the reaction mixture was reacted at 20°C for 24h. Post-treatment and purification, the crude product was recrystallized with isopropanol to obtain R-lipoic acid choline ester bromide as a light yellow solid (17.1g), with a yield of 95%. The reaction formula is:
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com