Efficient aprepitant preparation process
A preparation technology of aprepitant, which is applied in the field of high-efficiency preparation technology of aprepitant, can solve the problems of high cost, low yield, long reaction route, etc., and achieve low manufacturing cost, high yield and good drug effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
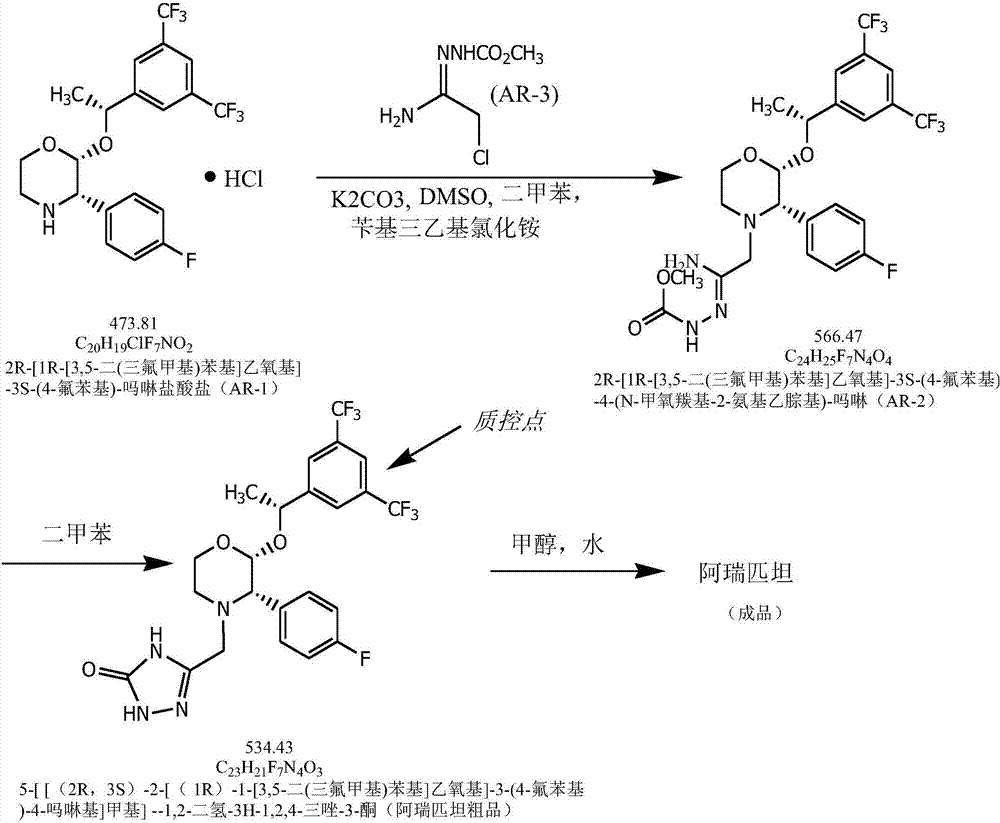
[0028] A high-efficiency preparation process of aprepitant of the present invention comprises the following process steps:
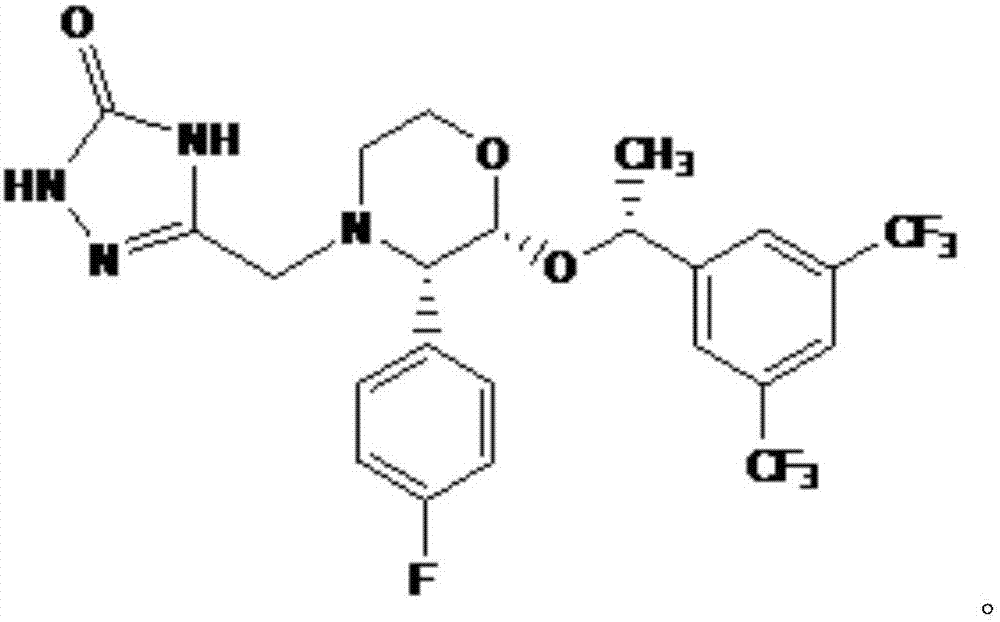
[0029] (1) With (2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-methoxy 2R-[1R-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3S-(4-fluorophenyl)-4-(N-methoxy Carbonyl-2-aminoethylhydrazone)-morpholine;
[0030] (2) Using 2R-[1R-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3S-(4-fluorophenyl)-4-(N-methoxycarbonyl-2- Aminoethylhydrazone)-morpholine prepares the crude product of aprepitant;
[0031] (3) Refining the crude product of aprepitant to obtain the finished product of aprepitant.
[0032] Wherein, the concrete process of described step (1) is:
[0033] 1) Fill the 100L reactor with nitrogen, add dimethyl sulfoxide, (2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy ]-3-(4-fluorophenyl)-morpholine hydrochloride and xylene, stir to dissolve, add potassium carbonate, stir at 20-25°C, then add (AR-3)2-(2-chloro-1- Iminoeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com