Method for preparing chimeric antigen receptor T cells
A chimeric antigen receptor and cell technology, applied in the field of biomedicine, can solve the problems of low lentivirus titer, low efficiency, and hinder the wide clinical application of T cells, and achieve the effect of increasing activation ability and improving infection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
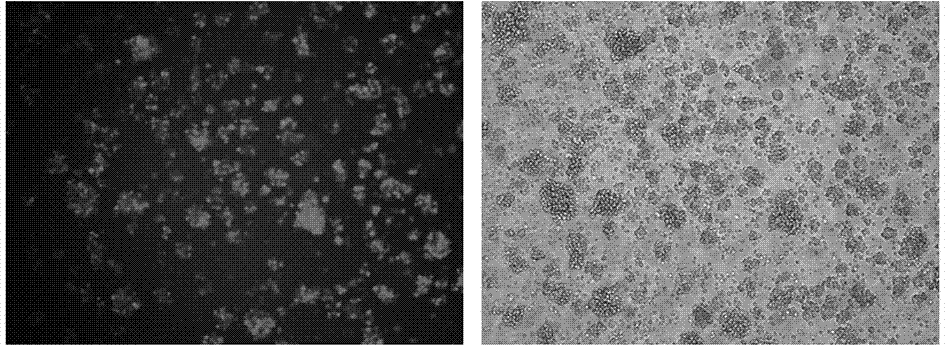
[0031] Prepare a 15cm dish and inoculate 5*10 6 293 cells, add complete medium (DMEM high glucose, 10% FBS), place at 37°C, 5% CO 2 incubator, overnight. Take out 100 μM polyetherimide (PEI) from the refrigerator, take out the plasmids (Lenti-GFP, pGP, pVSVG) from the refrigerator, and after thawing at room temperature, take out the PBS buffer and warm to room temperature; take 2 mL of PBS to a 6-well plate Prepare 3 wells in total, add 15 μg Lenti-EF1a-GFP, 3 μg pGP, 1.5 μg pVSVG respectively, pipette up and down to mix thoroughly, add 18 μL 100 μM polyetherimide to each well, and then add three The plasmid solution was added to the PBS well, immediately mixed up and down with a pipette, and allowed to stand at room temperature for 10 minutes to prepare the PEI / DNA complex. Add the above DNA / PEI complex dropwise into a 15cm petri dish, shake the petri dish gently, and mix well. Place the Petri dish at 37°C, 5% CO 2 After culturing for 5 hours, remove the medium containing...
Embodiment 2
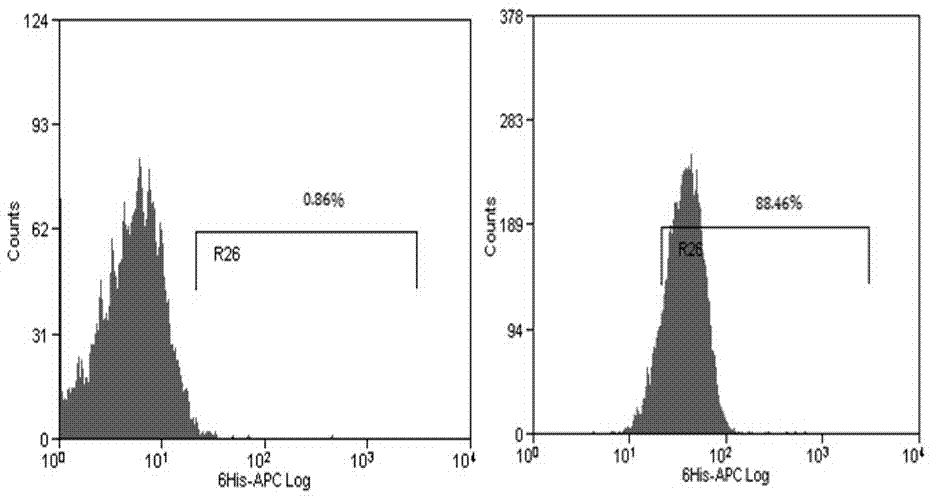
[0037] Based on the variable region of the antibody sequence targeting CD19, a chimeric antigen receptor is designed, which includes signal peptide, single-chain antibody sequence, transmembrane region and intracellular co-stimulatory signal region, and is synthesized by whole gene synthesis The chimeric antigen receptor sequence, and using the EcoRI-BamHI restriction site, the chimeric antigen receptor fragment was subcloned into the Lenti-puro lentiviral expression vector to prepare the Lenti-CD19-CAR lentiviral expression vector.
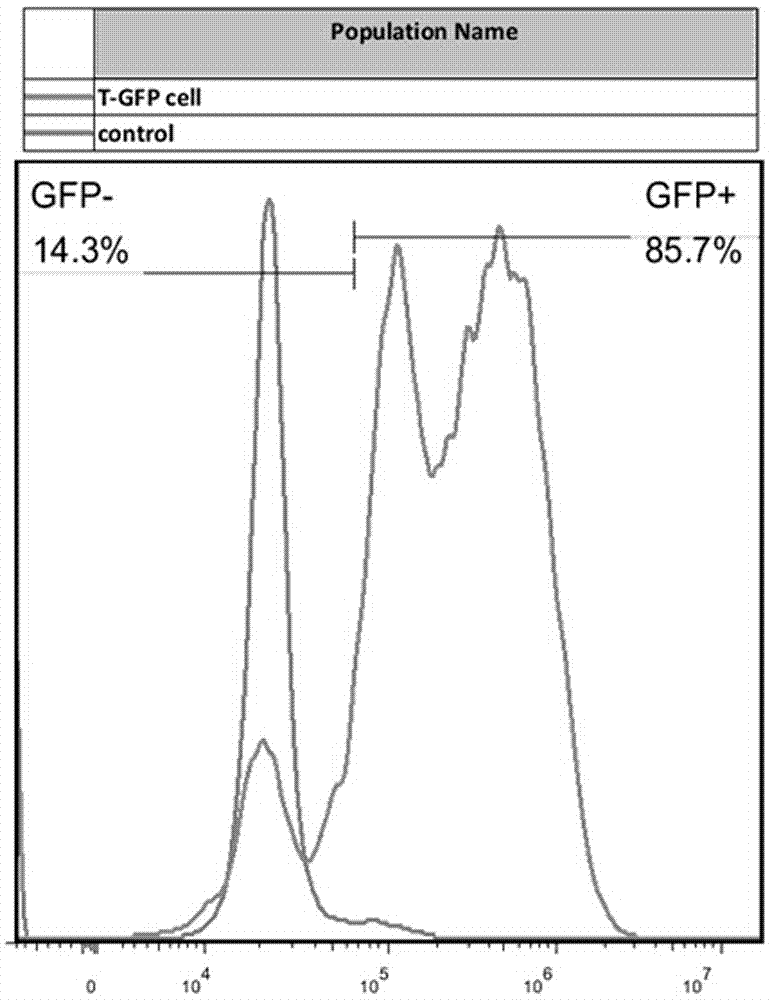
[0038] Prepare a 15cm dish and inoculate 5*10 6 293 cells, add complete medium (DMEM high glucose, 10% FBS), place at 37°C, 5% CO 2 incubator, overnight. Take out 100 μM polyetherimide from the refrigerator, take out the plasmids (Lenti-CD19-CAR, pGP, pVSVG) from the refrigerator, and after thawing at room temperature, take out the HBSS buffer and warm it to room temperature; take 2 mL of PBS into the 6-well plate One well, prepare 3 wells in t...
Embodiment 3
[0047]Prepare a 15cm dish and inoculate 5*10 6 293 cells, add complete medium (DMEM high glucose, 10% FBS), place at 37°C, 5% CO 2 incubator, overnight. Take out 100 μM polyetherimide from the refrigerator, take out the plasmids (Lenti-CD20-CAR, pGP, pVSVG) from the refrigerator, and after thawing at room temperature, take out the PBS buffer and warm to room temperature; Add 15 μg Lenti-CD20-CAR, 3 μg pGP, and 1.5 μg pVSVG to one well, mix well by pipetting up and down, then add 18 μL of 100 μM polyetherimide, immediately pipette up and down to mix, Let stand for 10 minutes to prepare PEI / DNA complexes. Add the above DNA / PEI complex dropwise into a 15cm petri dish, shake the petri dish gently, and mix well. Place the Petri dish at 37°C, 5% CO 2 After culturing for 10 hours, remove the medium containing the transfection reagent and replace it with fresh conditioned medium (DMEM high sugar, 5% FBS, sodium pyruvate, 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) )).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com