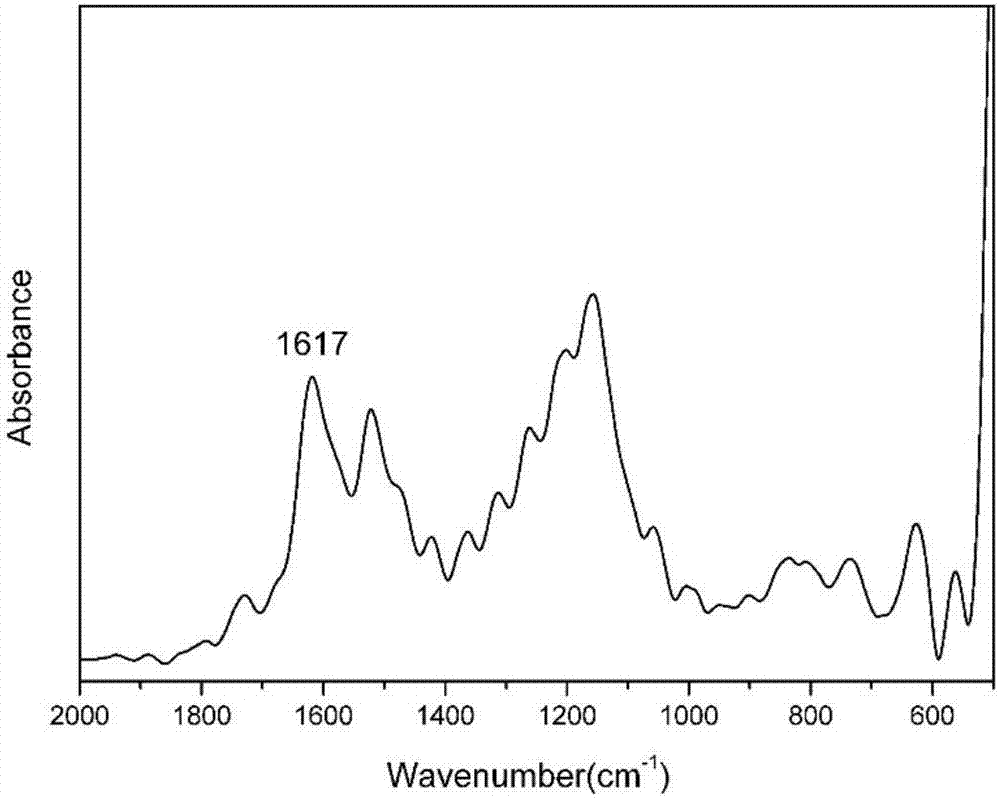
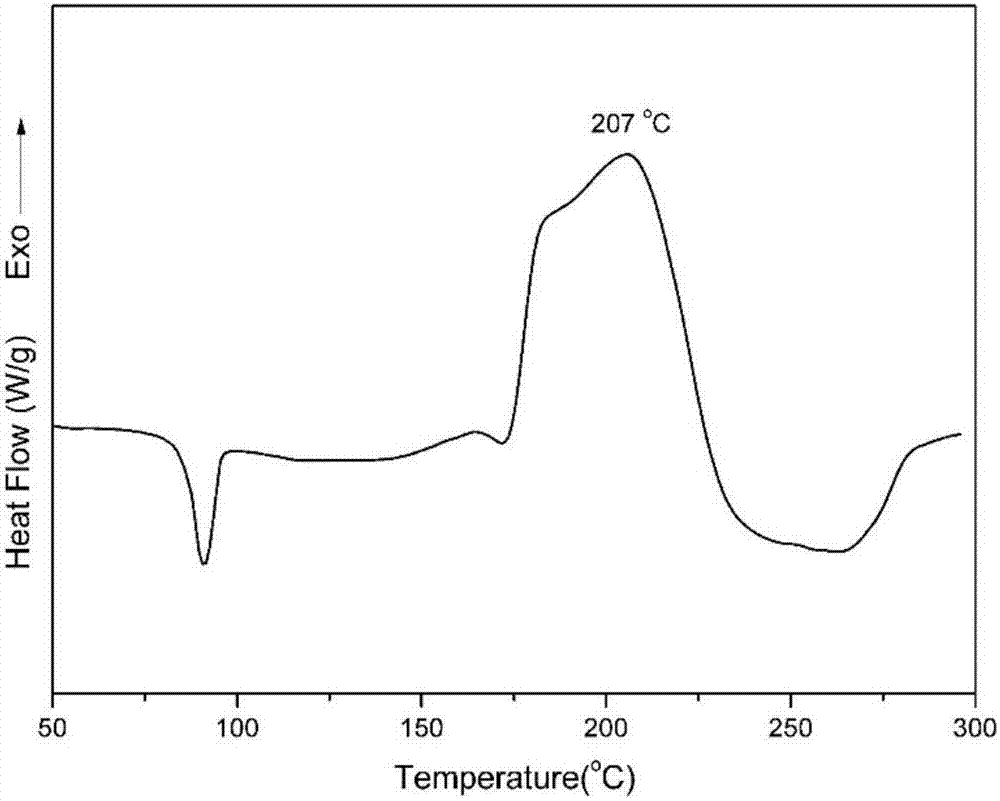
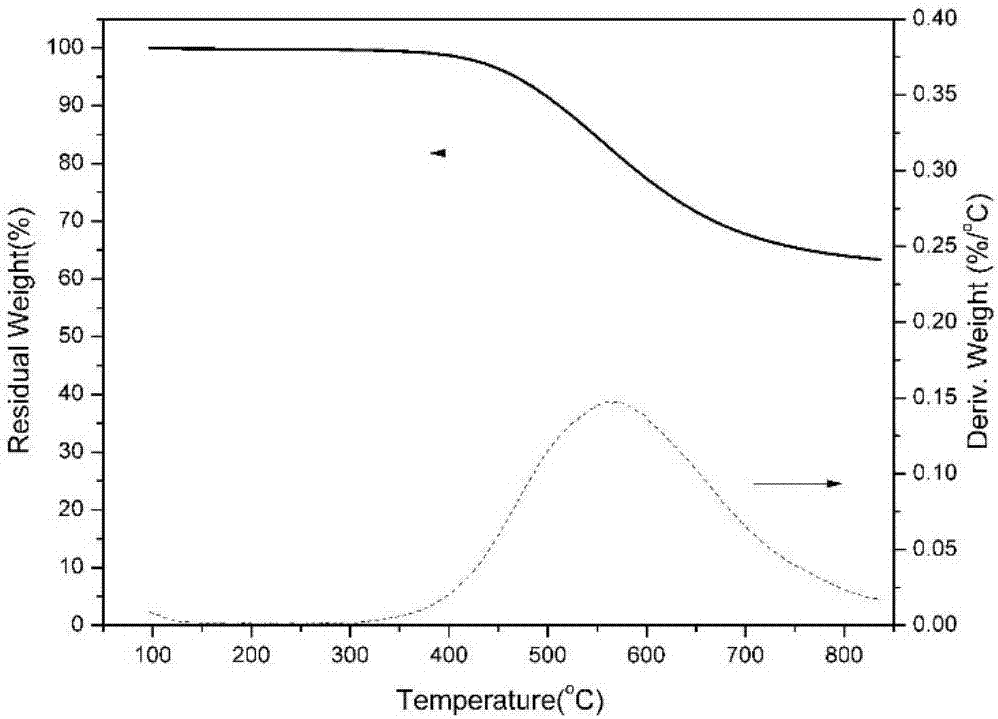
Benzoxazole resin and method for preparing same
A technology of benzoxazole and benzoxazine, which is applied in the field of thermosetting resin and its preparation, can solve the problems of easy ring-opening decomposition of benzoxazole ring and incomplete removal of phosphoric acid solvent, etc. Effects in simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The specific steps are:
[0043] (1) First add 21.82g of 2-aminophenol to 100mL of tetrahydrofuran solvent system, fully mix and dissolve in an ice bath, add 21.00g of trifluoroacetic anhydride dropwise after 15 minutes, and continue to react in an ice bath after adding 1.5 hours. After the reaction was finished, the solvent was removed by suspension evaporation, and the product was dissolved in ethyl acetate, washed three times with 5% sodium bicarbonate solution and deionized aqueous solution, and then the solvent was removed to obtain 17.64 g of the product o-trifluoroacetamide phenol. 82%. The reaction equation is as follows:
[0044]
[0045] (2) Weigh 10.26 g of o-trifluoroacetamide phenol, 4.96 g of diphenylmethanediamine, and 3.20 g of paraformaldehyde prepared in step (1) and mix them in 100 mL of xylene solvent, then heat up to 120° C., and the reaction time is 6 h. After stopping the reaction, wash with lye and evaporate the solvent to obtain a solid, a...
Embodiment 2
[0053] The reactant diphenylmethanediamine in the second step in Example 1 is replaced by 4,4'-diaminodiphenyl ether, the amount of reactant is changed accordingly, and other operating steps are the same as those in Example 1 .
[0054]In the second step reaction, the amount of the reactant was changed to: 10.26 g of o-trifluoroacetamide phenol obtained from the previous step reaction, 3.20 g of paraformaldehyde, and 5.01 g of 4,4'-diaminodiphenyl ether, to obtain Product 13.66g, yield 83%.
[0055] The specific chemical structure of the main chain type polybenzoxazine obtained is:
[0056]
[0057] Wherein the specific chemical structure of polybenzoxazole obtained is:
[0058]
[0059] The temperature of the benzoxazole resin obtained in this embodiment is 486° C. when the thermal weight loss is 5%, and the carbon residue rate at 800° C. is 69%. The dielectric constant of the benzoxazole resin is 2.3 at room temperature and 1 MHz.
Embodiment 3
[0061] The reactant diphenylmethanediamine in the second step in the embodiment 1 is replaced by 4,4'-diaminobenzophenone, and the amount of the reactant is changed accordingly, and other operation steps are the same as those in the embodiment 1 step.
[0062] In the second step reaction, the amount of the reactant was changed to: 10.26g of o-trifluoroacetamide phenol obtained from the previous step reaction, 3.20g of paraformaldehyde, 5.31g of 4,4'-diaminobenzophenone, 13.59 g of the product was obtained with a yield of 81%.
[0063] The specific chemical structure of the main chain type polybenzoxazine obtained is:
[0064]
[0065] Wherein the specific chemical structure of polybenzoxazole obtained is:
[0066]
[0067] The polybenzoxazole obtained in this example has a temperature of 501° C. when the thermal weight loss is 5%, and a carbon residue rate of 72% at 800° C., and the dielectric constant of the benzoxazole resin is 2.5 at room temperature and 1 MHz.
PUM
| Property | Measurement | Unit |
|---|---|---|
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com