Isoluminol derivative and preparation method and application thereof
A technology of isoluminol and derivatives, applied in the field of biological immunology detection, can solve the problems of sensitivity and detection limit, and achieve the effect of reducing impact, improving test sensitivity and detection limit, and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
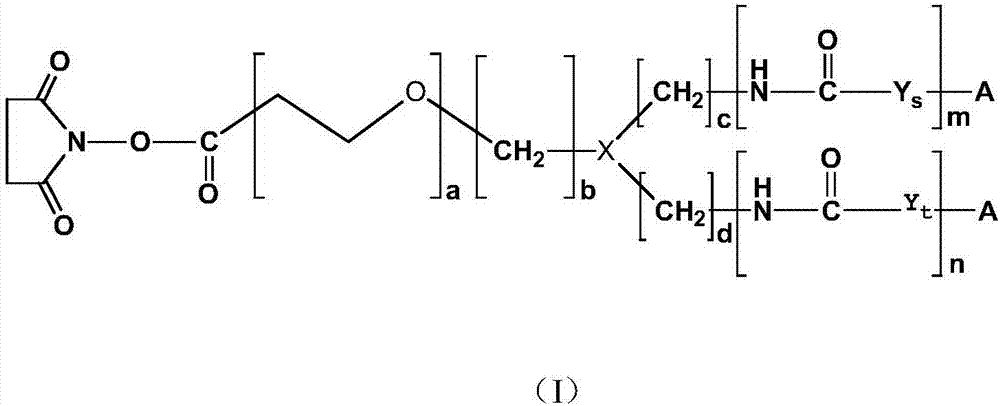
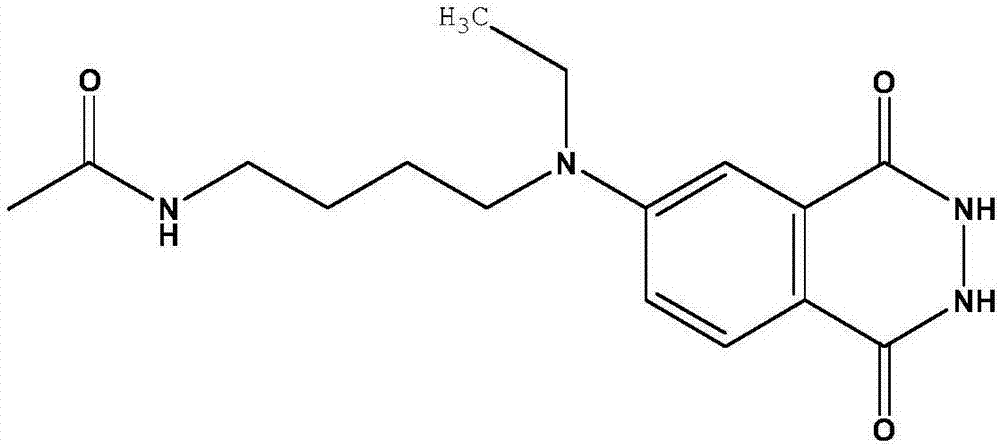
[0169] Preparation of isoluminol derivatives
[0170] 1) Synthesis of ABEI cyclic lactone;
[0171] Take 500mg of ABEI and 200mg of succinic anhydride, dissolve in 30ml of anhydrous DMSO solvent, pass through argon protection, stir in a water bath at 50°C for 30min, transfer to room temperature and avoid light, continue to react for 120min, add 400mg of EDC, stir at room temperature for 24h, and end the reaction . Ethyl acetate was added for extraction, washed with ultrapure water, dried over anhydrous sodium sulfate, spun to dryness, and separated on a silica gel column to obtain ABEI cyclic lactone.
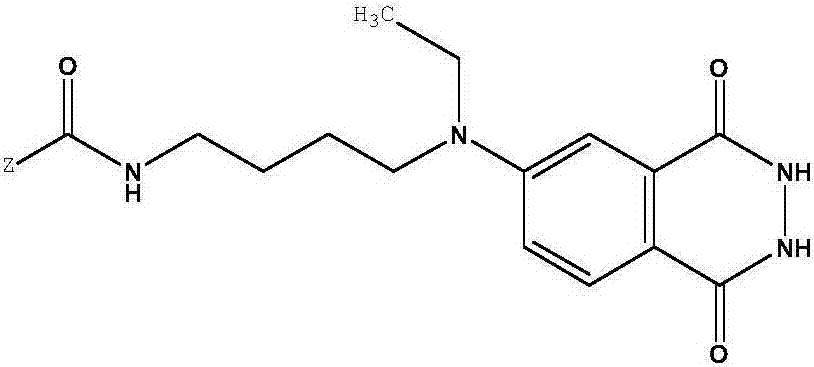
[0172] 2) Preparation of the first intermediate product
[0173] Dissolve 100mg of ABEI cyclic lactone and 34mg of 3-(N,N-diaminopropylamino)propionic acid obtained in step 1) in 5ml of anhydrous DMSO, and react in the dark at 37°C for 4h under the protection of argon, at room temperature Let stand overnight. Add a large amount of ethyl acetate for extraction, wash with dil...
Embodiment 2
[0179] Preparation of isoluminol derivatives
[0180] 1) Synthesis of ABEI cyclic lactone;
[0181] Take 500mg of ABEI and 290mg of adipic anhydride, dissolve in 30ml of anhydrous DMF solvent, pass through argon protection, stir in a water bath at 40°C for 50min, transfer to room temperature to avoid light and continue to react for 3 hours, add 440mg of DCC, stir at room temperature for 24h, end reaction. Ethyl formate was added for extraction, washed with ultrapure water, dried over anhydrous magnesium sulfate, evaporated to dryness, and separated on a silica gel column to obtain ABEI cyclic lactone.
[0182] 2) Preparation of the first intermediate product
[0183] Dissolve 100 mg of ABEI cyclic lactone and 21 mg of 2,4-diaminovaleric acid obtained in step 1) in 5 ml of anhydrous DMF, and react at 37°C in the dark for 3 hours under the protection of argon, and stand at room temperature overnight. Add a large amount of ethyl formate for extraction, wash with dilute hydroch...
Embodiment 3
[0189] Preparation of isoluminol derivatives
[0190] 1) Synthesis of ABEI cyclic lactone;
[0191] Take 500mg of ABEI and 270mg of glutaric anhydride, dissolve in 30ml of anhydrous DMAc solvent, pass through argon protection, stir in a water bath at 60°C for 20min, transfer to room temperature and avoid light, continue to react for 60min, add 450mg of EDC, stir at room temperature for 24h, and end the reaction . Ethyl acetate was added for extraction, washed with ultrapure water, dried over anhydrous sodium sulfate, spun to dryness, and separated on a silica gel column to obtain ABEI cyclic lactone.
[0192] 2) Preparation of the first intermediate product
[0193] Dissolve 100 mg of ABEI cyclic lactone and 25 mg of 3,5-diaminovaleric acid obtained in step 1) in 5 ml of anhydrous DMAc, and react at 37° C. in the dark for 4 h under the protection of argon, and stand at room temperature overnight. Add a large amount of ethyl acetate for extraction, wash with dilute hydrochlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com