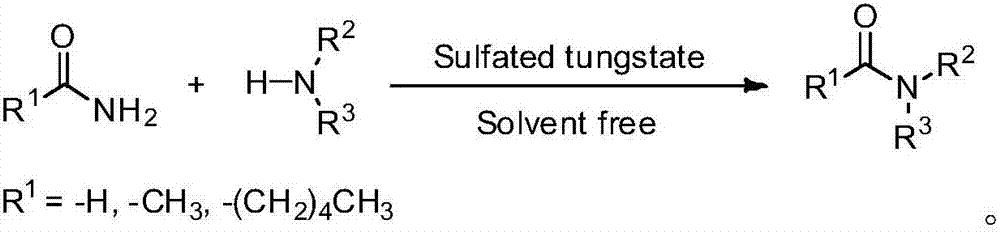
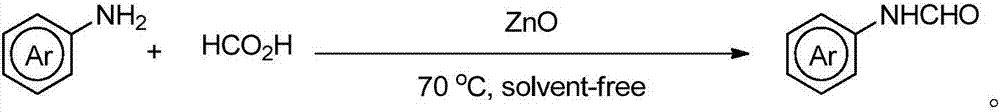Novel method for synthesizing N-substitute amide derivative
A technology of amide derivatives and a new method, which is applied in the preparation of organic compounds, the formation/introduction of amide groups, the preparation of carboxylic acid amides, etc., can solve the problems of high reaction temperature, limited substrate range, cumbersome operation process, etc. Simple operation, easy product separation and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] At room temperature, add 0.2 mmol of the compound represented by formula (I-1) and 1 ml of formamide into a reaction tube equipped with a stirring magnet, stir and raise the temperature to 120° C., and react at this temperature for 15 h.
[0038] After the reaction, the reaction solution was cooled to room temperature, 15ml of ethyl acetate and 15ml of water were added, the organic layer was washed with 15ml of saturated ammonium chloride solution and 15ml of saturated sodium chloride solution, dried over anhydrous sodium sulfate for 1 hour, and covered with a layer of Silica gel funnel was used for filtration, and then the solvent was distilled off under reduced pressure, and the residue was purified by thin-layer chromatography silica gel plate (20cm×20cm) to obtain the target product represented by formula (III-1).
[0039] After calculation, the yield of the target product represented by formula (III-1) is 99%.
[0040] NMR data: 1 H NMR (500MHz, CDCl ...
Embodiment 2
[0043]
[0044] At room temperature, add 0.2 mmol of the compound represented by formula (I-2) and 1 ml of formamide into a reaction tube equipped with a stirring magnet, stir and raise the temperature to 120° C., and react at this temperature for 15 h.
[0045] After the reaction, the reaction solution was cooled to room temperature, 15ml of ethyl acetate and 15ml of water were added, the organic layer was washed with 15ml of saturated ammonium chloride solution and 15ml of saturated sodium chloride solution, dried over anhydrous sodium sulfate for 1 hour, and covered with a layer of Silica gel funnel was used for filtration, and then the solvent was distilled off under reduced pressure, and the residue was purified by thin-layer chromatography silica gel plate (20cm×20cm) to obtain the target product represented by formula (III-2).
[0046] After calculation, the yield of the target product represented by formula (III-2) is 74%.
[0047] NMR data: 1 H NMR (500MHz, CDCl 3 ...
Embodiment 3
[0050]
[0051] At room temperature, add 0.2 mmol of the compound represented by formula (I-3) and 1 ml of formamide into a reaction tube equipped with a stirring magnet, stir and raise the temperature to 120° C., and react at this temperature for 15 h.
[0052] After the reaction, the reaction solution was cooled to room temperature, 15ml of ethyl acetate and 15ml of water were added, the organic layer was washed with 15ml of saturated ammonium chloride solution and 15ml of saturated sodium chloride solution, dried over anhydrous sodium sulfate for 1 hour, and covered with a layer of Silica gel funnel was used for filtration, and then the solvent was distilled off under reduced pressure, and the residue was purified by thin-layer chromatography silica gel plate (20cm×20cm) to obtain the target product represented by formula (III-3).
[0053] After calculation, the yield of the target product represented by formula (III-3) is 55%.
[0054] NMR data: 1 H NMR (500MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com