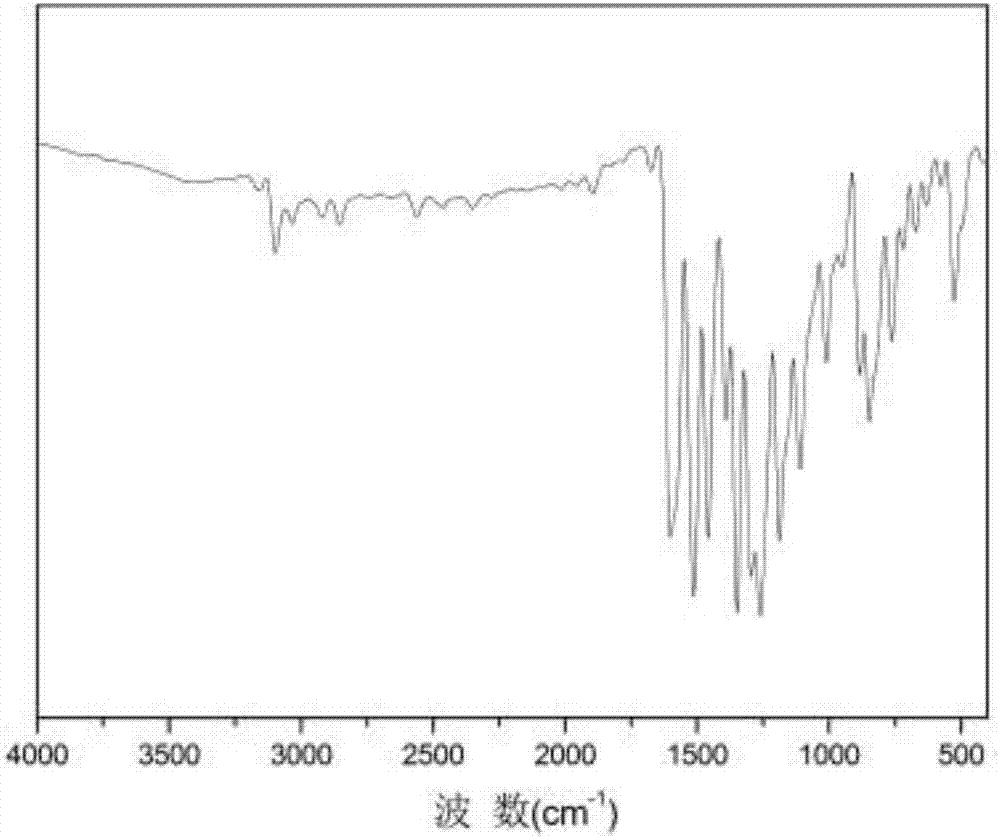
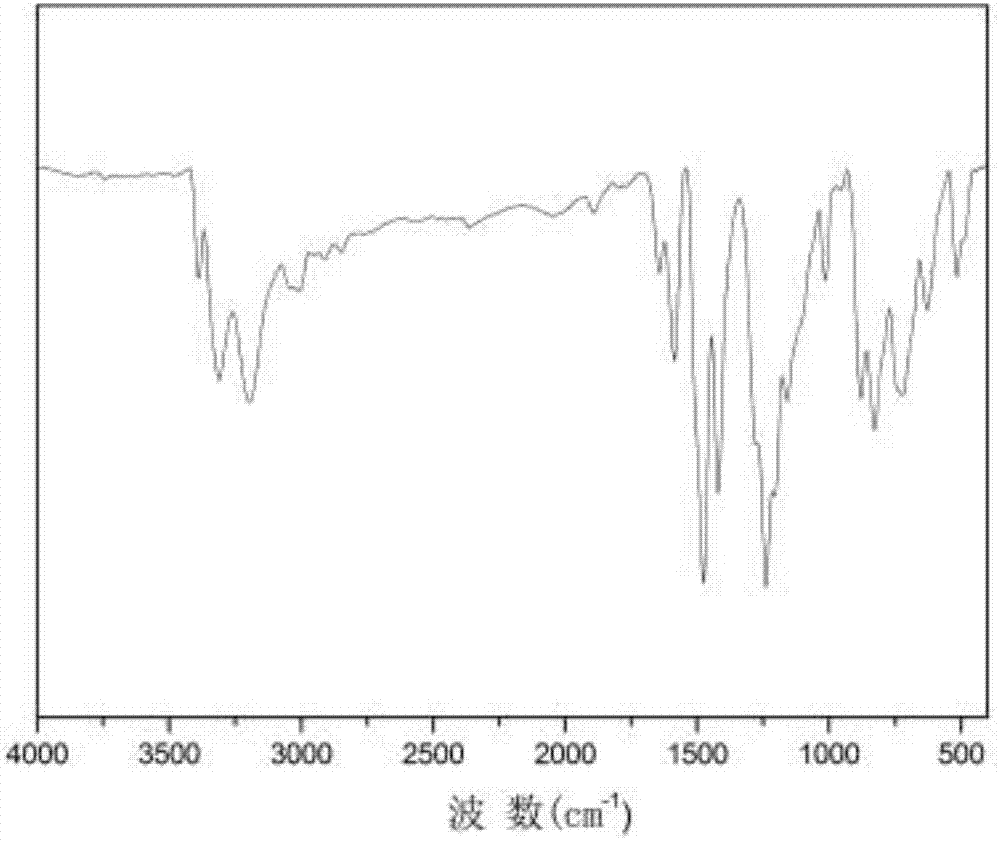
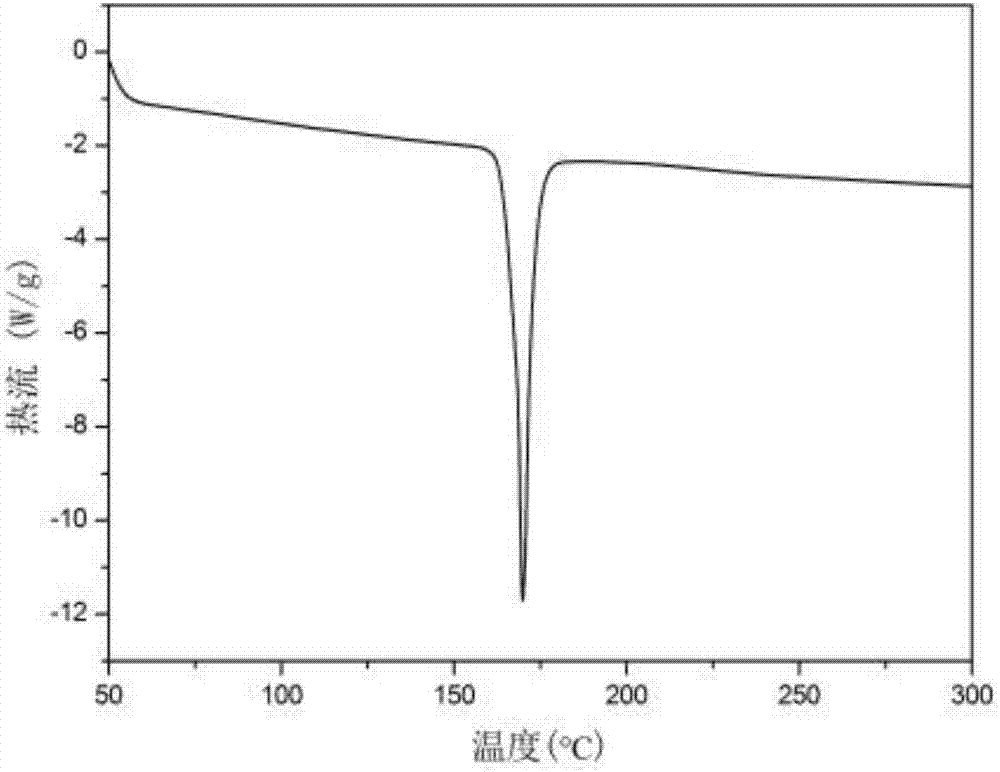
Pyridazine-group-containing diamine monomer and preparation method and application thereof
A technology of diamine monomer and pyridazineoxy group, which is applied in the field of diamine monomer containing pyridazine structure and its preparation, can solve the problems of poor optical transmission of polyimide materials, and avoid the difficulty of post-reduction treatment , reasonable design, reduce the effect of conjugation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Under room temperature and nitrogen protection conditions, 54.40mmol (11.99g) 4,4'-dihydroxydiphenylmethane, 130.56mmol (20.83g) 3-chloro-2-nitropyridazine and 130.56mmol (18.04 g) Potassium carbonate was dissolved in 1935mmol (180ml) N,N'-dimethylacetamide, the total mass fraction of solids in the system was 10% to 50%, then heated to 60°C, and reacted for 6 hours; after cooling down to room temperature, The reactant is poured into a sodium hydroxide solution with a mass fraction of 5%, filtered, and the resulting solid is repeatedly washed with deionized water until the washing liquid is neutral to obtain 15 g of dinitro crude product; Dissolve 6g of activated carbon in 861mmol (80ml) of N,N'-dimethylacetamide, heat to 70-90°C, filter after 30 minutes, add water dropwise to the filtrate until the solution is saturated, filter, and place the obtained solid in vacuum at 80°C Treated for 8 hours to obtain 4,4'-bis(6-nitro-3-pyridazinyloxy)diphenylmethane containing a...
Embodiment 2
[0038] (1) Under room temperature and nitrogen protection conditions, 54.40mmol (11.99g) 3,3'-dihydroxydiphenylmethane, 146.88mmol (23.43g) 3-chloro-2-nitropyridazine and 146.88mmol (20.71 g) Potassium carbonate was dissolved in 1613mmol (150ml) N,N'-dimethylacetamide, the total mass fraction of solids in the system was 10% to 50%, then heated to 60°C, and reacted for 6 hours; after cooling down to room temperature, The reactant was poured into a sodium hydroxide solution with a mass fraction of 5%, filtered, and the obtained solid was repeatedly washed with deionized water until the washing liquid was neutral, and 12 g of dinitro crude product was obtained; the dinitro crude product was Dissolve 6g of activated carbon in 964mmol (90ml) N,N'-dimethylacetamide, heat to 70-90°C, filter after 30 minutes, add water dropwise to the filtrate until the solution is saturated, filter, and vacuum the obtained solid at 80°C Treated for 8 hours to obtain 3,3'-bis(6-nitro-3-pyridazinyloxy)...
Embodiment 3
[0043] (1) Under room temperature and nitrogen protection conditions, 54.40mmol (15.14g) 3-hydroxy-6-(4-hydroxyphenoxy)biphenyl, 163.20mmol (26.03g) 3-chloro-2-nitropyridine Dissolve oxazine and 163.20mmol (23.01g) of potassium carbonate in 1720mmol (160ml) of N,N'-dimethylacetamide, the total mass fraction of solids in the system is 10% to 50%, then heat to 60°C and react for 6 hours After being down to room temperature, the reactant is poured into a mass fraction of 5% sodium hydroxide solution, filtered, and the gained solid is repeatedly washed with deionized water until the washing liquid is neutral, and 14 g of dinitro crude product is obtained; The dinitro crude product and 8.4g of activated carbon were dissolved in 1286mmol (120ml) N,N'-dimethylacetamide, heated to 70-90°C, filtered after 30 minutes, added water dropwise to the filtrate until the solution was saturated, and filtered , the resulting solid was vacuum-treated at 80°C for 8 hours to obtain 3-(6-nitro-3-pyr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com